
Global Pediatric Perfusion Product Market – Overview
Pediatric perfusion devices are vital to the field of pediatric cardiac surgery because they offer young patients undergoing intricate cardiovascular procedures the vital assistance they need. With the help of these specialist goods, cardiopulmonary bypass (CPB) can be performed more easily and successfully during surgery by maintaining proper circulation and oxygenation. A variety of tools and equipment designed specially to satisfy the special physiological requirements of young patients are referred to as pediatric perfusion products. With the patient’s circulation momentarily redirected away from the heart, these devices enable surgeons to operate on the heart and blood arteries while maintaining ideal blood flow, oxygenation, and temperature control during cardiac surgery. The heart-lung machine (also called the cardiopulmonary bypass machine), oxygenators, blood pumps, cannulas, filters, tubing sets, and monitoring systems are essential parts of pediatric perfusion devices. The main device that controls blood flow and gas exchange outside the body during surgery is the heart-lung machine. Blood pumps keep circulation going by reintroducing oxygenated blood into the patient’s body, while oxygenators help the blood’s natural exchange of carbon dioxide and oxygen. The patient’s blood arteries are accessed using cannulas to link them to the bypass circuit, and air bubbles and debris are removed from the blood using filters to avoid embolisms.
Pediatric cardiac surgeries, such as those involving the repair of congenital heart defects, the correction of cardiac anomalies, and heart transplant procedures, all make use of pediatric perfusion products. During surgery, these goods are essential for supporting the patient’s circulation, preserving organ perfusion, and safeguarding the patient’s critical organs. Furthermore, extracorporeal membrane oxygenation (ECMO) therapy, a life-saving measure for kids with severe respiratory or cardiac failure, uses pediatric perfusion products. They enable surgeons to perform intricate procedures with precision and confidence, minimizing the risk of complications and maximizing patient outcomes.
Global Pediatric Perfusion Product Market- Competitive Insights
Sep 1st, 2022 – CHS USA Inc., a company of Canadian Hospital Specialties Limited, completed the acquisition of SandBox Medical LLC. This acquisition will increase CHS’s presence in the Neonatal Intensive Care Unit and Pediatric market while providing innovative products for premature babies and full-term newborns. SandBox Medical and CHS are both known in the healthcare industry for high-quality and reliable products.
Some of the Key Players in the Global Pediatric Perfusion Product Markets Include-
Global Pediatric Perfusion Product Market- Growth Drivers
Technology innovation is the primary factor propelling the Pediatric Perfusion Product industry’s expansion. The safety and effectiveness of pediatric cardiac procedures have been greatly enhanced by technological advancements in medical devices, including sophisticated oxygenators, compact cardiopulmonary bypass systems, and pediatric-specific cannulation techniques. The need for pediatric perfusion products is being driven by technical advancements that allow surgeons to conduct complex procedures with more precision, fewer need for blood transfusions, and a lower risk of neurological problems. Another significant driver propelling the Pediatric Perfusion Product market upward is the rising incidence of congenital heart abnormalities and other pediatric cardiovascular diseases. Congenital heart abnormalities are among the most common birth defects, affecting about 1% of babies worldwide. An increasing number of cases are being identified early and require surgical procedures as awareness and diagnostic skills grow. The need for perfusion solutions specifically designed to meet the physiological requirements of pediatric patients is being driven by the expanding patient pool. Increased awareness regarding the significance of pediatric perfusion products among medical practitioners and guardians is encouraging market expansion. To maximize surgical results and reduce complications, healthcare practitioners are releasing more and more about how important it is to use personalized perfusion systems made especially for pediatric patients.
Global Pediatric Perfusion Product Market- Restraints
Manufacturers have limited potential for earnings because the market for pediatric perfusion devices is smaller than that of adult perfusion goods. Furthermore, several competitors are vying for market share in the fragmented pediatric perfusion industry. In particular, smaller firms without well-established brand recognition may face pricing pressure and decreased profitability as a result of this fragmentation. As pediatric cardiac surgery is intrinsically complicated, specific knowledge and tools are needed. Pediatric patients’ small stature and distinct anatomy present difficulties for perfusionists and surgeons, calling for the creation of customized perfusion supplies. To overcome these obstacles and guarantee the security and effectiveness of their goods for use in pediatric cardiac surgery, manufacturers must make research and development investments. Given the vulnerability of children, pediatric perfusion products must pass stringent regulatory review and approval processes. The European Medicines Agency (EMA) in Europe and the Food and Drug Administration (FDA) in the United States have strict regulations that manufacturers must follow. It can take a lot of time and money to meet these standards, which might delay product launches and raise development expenses.
Global Pediatric Perfusion Product Market- Opportunities
The creation of innovative pediatric perfusion products is made possible by the fast pace at which medical device technology is developing. Innovative products like compact cardiopulmonary bypass machines, integrated monitoring systems, and enhanced oxygenators made especially for young patients can be introduced by businesses that invest in research and development. Pediatric patients require specific perfusion products that are suited to their demands due to their distinct physiological requirements that are different from those of adults. Businesses that concentrate on modifying and adjusting current technologies to suit pediatric patients’ anatomy and physiology can capitalize on this niche market segment and gain a competitive advantage. By collaborating with local healthcare providers, providing accessible and reasonably priced pediatric perfusion products, and funding educational campaigns to increase public awareness of the significance of prompt intervention and treatment of pediatric cardiac conditions, businesses can establish a foothold in these areas. The need for perfusion methods that work with less invasive surgical techniques in pediatric cardiac surgery is rising as these procedures become more popular. There are opportunities for businesses to create perfusion systems that are suited to minimally invasive techniques, allowing doctors to execute difficult cardiac surgeries with lower rates of complications and quicker recovery periods.
Global Pediatric Perfusion Product Market- Geographical Insights
The market for Pediatric Perfusion Products is segmented into regions such as North America, Latin America, Europe, Asia-Pacific, the Middle East, and Africa. In North America, particularly in the United States and Canada, the market for pediatric perfusion products is characterized by advanced healthcare infrastructure, high healthcare expenditure, and a strong emphasis on pediatric cardiac care. The region boasts leading pediatric cardiac centers, specialized hospitals, and academic institutions dedicated to pediatric cardiology and cardiac surgery, driving demand for advanced perfusion technologies tailored to pediatric patients. Latin American market participants concentrate on offering affordable and portable perfusion equipment, training programs for medical professionals, and cooperative initiatives with government agencies and non-profit organizations to improve access to pediatric cardiac care in underserved areas. These cost-effective perfusion solutions are tailored to the needs of pediatric patients. Market players in Europe focus on developing innovative perfusion technologies, enhancing patient outcomes, and addressing unmet needs in pediatric cardiac care through collaborative research, clinical trials, and technology partnerships with academic and clinical institutions.
Global Pediatric Perfusion Product Market- Key Development
Aug 3, 2023 – Long-term normothermic ex-situ perfusion of human split livers for over a week was achieved, demonstrating the feasibility of extending survival beyond 7 days.
Research Methodology: Aspects
Market research is a crucial tool for organizations aiming to navigate the dynamic landscape of customer preferences, business trends, and competitive landscapes. At Cognizance Market Research, acknowledging the importance of robust research methodologies is vital to delivering actionable insights to our clientele. The significance of such methodologies lies in their capability to offer clarity in complexity, guiding strategic management with realistic evidence rather than speculation. Our clientele seek insights that excel superficial observations, reaching deep into the details of consumer behaviours, market dynamics, and evolving opportunities. These insights serve as the basis upon which businesses craft tailored approaches, optimize product offerings, and gain a competitive edge in an ever-growing marketplace.
The frequency of information updates is a cornerstone of our commitment to providing timely, relevant, and accurate insights. Cognizance Market Research adheres to a rigorous schedule of data collection, analysis, and distribution to ensure that our reports reflect the most current market realities. This proactive approach enables our clients to stay ahead of the curve, capitalize on emerging trends, and mitigate risks associated with outdated information.
Our research process is characterized by meticulous attention to detail and methodological rigor. It begins with a comprehensive understanding of client objectives, industry dynamics, and research scope. Leveraging a combination of primary and secondary research methodologies, we gather data from diverse sources including surveys, interviews, industry reports, and proprietary databases. Rigorous data analysis techniques are then employed to derive meaningful insights, identify patterns, and uncover actionable recommendations. Throughout the process, we remain vigilant in upholding the highest standards of data integrity, ensuring that our findings are robust, reliable, and actionable.
Key phases involved in in our research process are mentioned below:

Understanding Clients’ Objectives:
Extensive Discussions and Consultations:
Industry and Market Segment Analysis:
Target Audience Understanding:
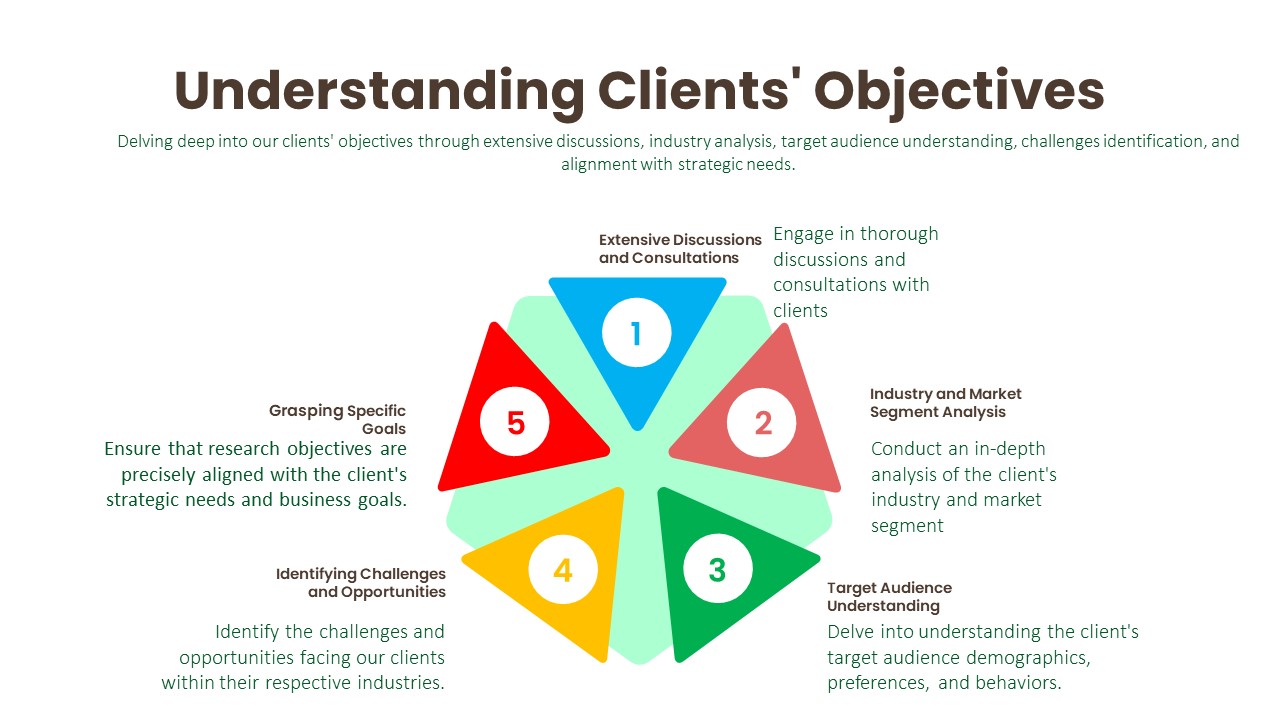
Identifying Challenges and Opportunities:
Grasping Specific Goals:
Data Collection:
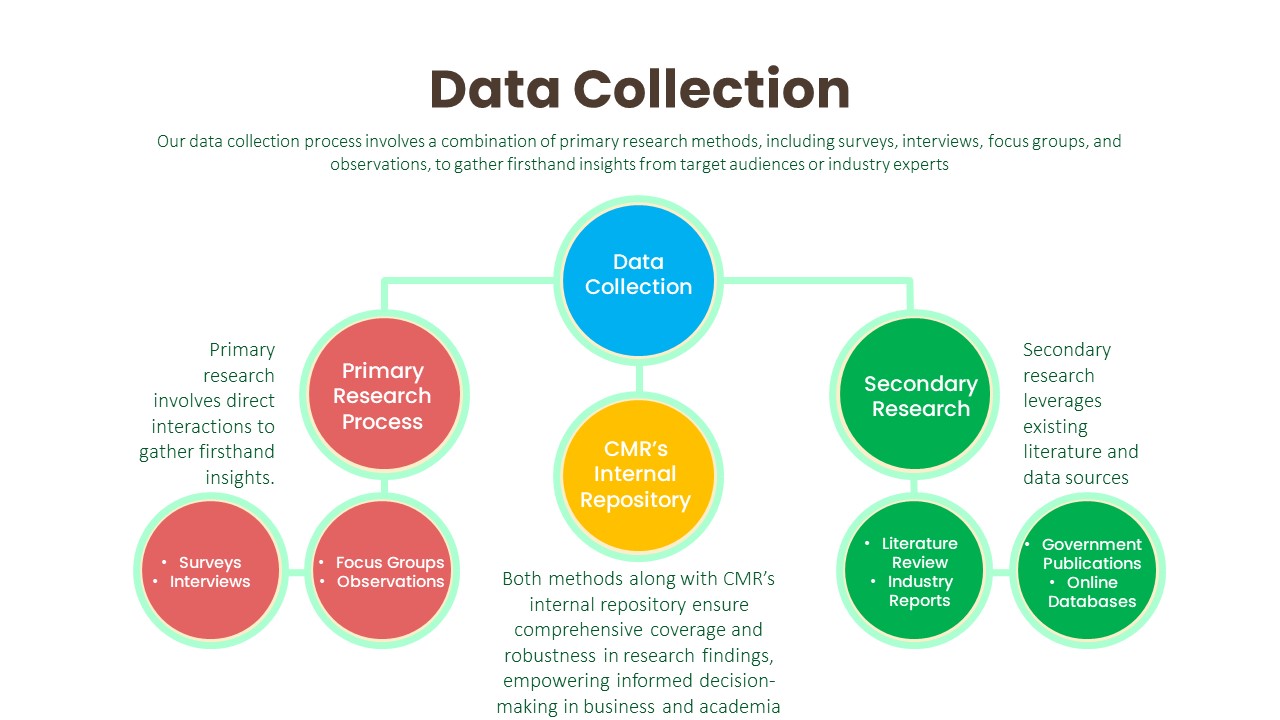
Primary Research Process:
Secondary Research Process:
Data Analysis:
The data analysis phase serves as a critical juncture where raw data is transformed into actionable insights that inform strategic decision-making. Through the utilization of analytical methods such as statistical analysis and qualitative techniques like thematic coding, we uncover patterns, correlations, and trends within the data. By ensuring the integrity and validity of our findings, we strive to provide clients with accurate and reliable insights that accurately reflect the realities of the market landscape.

Transformation of Raw Data:
Utilization of Analytical Methods:
Statistical Analysis:
Qualitative Analysis Techniques:
Integrity and Validity Maintenance:
Data Validation:
The final phase of our research methodology is data validation, which is essential for ensuring the reliability and credibility of our findings. Validation involves scrutinizing the collected data to identify any inconsistencies, errors, or biases that may have crept in during the research process. We employ various validation techniques, including cross-referencing data from multiple sources, conducting validity checks on survey instruments, and seeking feedback from independent experts or peer reviewers. Additionally, we leverage internal quality assurance protocols to verify the accuracy and integrity of our analysis. By subjecting our findings to rigorous validation procedures, we instill confidence in our clients that the insights they receive are robust, reliable, and trustworthy.
Importance of Data Validation:
Scrutiny of Collected Data:
Validation Techniques:
Internal Quality Assurance Protocols:
We can customize every report – free of charge – including purchasing stand-alone sections or country-level reports
We help clients to procure the report or sections of the report at their budgeted price. Kindly click on the below to avail

Cognizance market research is continuously guiding customers around the globe towards strategies for transformational growth. Today, businesses have to innovate more than ever before, not just to survive, but to succeed in the future

© 2023 All rights Reserved. Cognizance Market Research