
Global Invitro Toxicology Testing Market – Overview
Invitro toxicology testing market refers to the sector dedicated to the development, production, and provision of methods and technologies for assessing the toxicity of substances using non-animal models, such as cell cultures, tissues, or biochemical systems, conducted in controlled laboratory environments. This market includes a wide range of products and services aimed at evaluating the safety and potential risks of chemicals, pharmaceuticals, cosmetics, and other substances while minimizing the need for animal testing. It serves various industries by evaluating the safety of substances like pharmaceuticals, cosmetics, chemicals, and food additives. It helps in drug development, consumer safety, environmental protection, and regulatory compliance while reducing reliance on animal testing. It’s employed in pharmaceuticals to screen drug candidates, in cosmetics to ensure product safety, in chemicals to evaluate industrial compounds, in food to test additives, in environmental monitoring to assess pollutants, and for regulatory compliance to provide data for governmental agencies, all while reducing reliance on animal testing.
The World Health Organization (WHO) sets international standards for toxicology testing, ensuring product safety and environmental sustainability. It collaborates with regulatory agencies, providing expertise for public health initiatives on toxicology and chemical safety. In vitro tests are based on primary cells, immortalized (e.g., SV40 transformation) and cancer-derived cell lines, stem cells, or reconstituted tissue cultures, it is important to have in vitro systems that adequately mimic key events of the in vivo mechanisms of action triggered in humans upon exposure to a toxic compound.
Global Invitro Toxicology Testing Market – Competitive Insight-
On January 18, 2023, Eurofins Scientific expanded its presence in India with the establishment of a new, fully equipped, state-of-the-art laboratory campus in Genome Valley, Hyderabad. The lab will support pharma and biotech companies in the areas of synthetic organic chemistry, analytical R&D, bioanalytical services, in vivo pharmacology, safety toxicology, and formulation R&D.
On March 9, 2023, Agilent Technologies, Inc. (US) acquired e-MSion (US). Through this acquisition, Agilent will integrate the e-MSion’s ExD cell into its portfolio of advanced workflows, instruments, and analytical solutions for biotherapeutic characterization and development.
On April 20, 2023, Gentronix, Ltd expanded its laboratory to fulfill the growing demand.
Some of the Key Players in the Global Invitro Toxicology Testing Markets include –
Global Invitro Toxicology Testing Market – Growth Drivers
Increasing awareness and ethical concerns surrounding the use of animals in scientific research, particularly in toxicology testing, have catalyzed a notable shift towards in vitro methods. This transition is driven by a collective recognition of the ethical implications and welfare considerations associated with animal experimentation. In response to these concerns, there is a growing demand for alternative testing methods that do not rely on the use of animals. Advancements in cell culture, high-throughput screening, and computational modeling are improving the accuracy, efficiency, and cost-effectiveness of in vitro toxicology testing. Demand for cosmetics and personal care products is driving manufacturers to invest in in vitro testing for safety and compliance. In vitro testing methods present significant cost savings compared to traditional animal testing, as they require less time and fewer resources, and alleviate ethical concerns associated with animal experimentation. These advantages make them increasingly appealing to industries and regulatory bodies alike. As a result, there is a growing adoption of in vitro approaches across various sectors, driven by the recognition of their efficiency, effectiveness, and alignment with ethical standards.
Global Invitro Toxicology Testing Market – Restraints
The Main Challenge faced by Invitro toxicology testing Market is a regulatory expense, regulatory acceptance of in vitro methods can vary, posing challenges for widespread adoption and market growth. Maintaining consistency and reproducibility across various in vitro assays and platforms poses a significant challenge in the field of toxicology testing. The diversity of methodologies, cell lines, culture conditions, and assay protocols utilized in different laboratories can lead to variations in results, affecting the reliability and comparability of data. Despite advancements, accurately modeling certain toxicological endpoints, like complex systemic or long-term exposure effects, remains challenging with current in vitro technologies. One of the primary reasons for this challenge is the lack of standardized protocols and quality control measures across the industry.
Global Invitro Toxicology Testing Market – Opportunities
The continuous evolution and innovation in cell culture techniques, organ-on-a-chip models, and high-throughput screening methods represent a significant opportunity for advancing the field of in vitro toxicology testing. These advancements not only enhance the accuracy and predictive capabilities of in vitro testing but also open new avenues for more sophisticated and comprehensive toxicity assessments. Collaborations between academia, industry, and regulatory bodies can drive innovation and facilitate the development of standardized protocols and best practices in in vitro testing. As regulatory agencies increasingly recognize and accept in vitro methods, companies have the opportunity to obtain market approval for their products more quickly and affordably. The emerging markets in Asia-Pacific and Latin America offer untapped opportunities for the expansion of in vitro testing services and technologies. These regions are experiencing rapid economic growth, urbanization, and industrialization, leading to increased demand for safer and more reliable products across various sectors, including pharmaceuticals, cosmetics, chemicals, and food.
Global Invitro Toxicology Testing Market – Geographical Insight
The market for Invitro toxicology testing is segmented into regions such as North America, Latin America, Europe, Asia-Pacific, the Middle East & Africa. North America is the largest market for Invitro toxicology testing due to well-established healthcare infrastructure facilities and widespread diagnosis and treatment. Advanced healthcare systems support timely diagnosis and treatment. Regulatory frameworks influence market dynamics and product approvals. A large population base, particularly in countries like China and India, provides a substantial market. Increasing healthcare expenditure and improving healthcare infrastructure enhance market growth prospects.
Global Invitro Toxicology Testing Market – Key Development
Research Methodology: Aspects
Market research is a crucial tool for organizations aiming to navigate the dynamic landscape of customer preferences, business trends, and competitive landscapes. At Cognizance Market Research, acknowledging the importance of robust research methodologies is vital to delivering actionable insights to our clientele. The significance of such methodologies lies in their capability to offer clarity in complexity, guiding strategic management with realistic evidence rather than speculation. Our clientele seek insights that excel superficial observations, reaching deep into the details of consumer behaviours, market dynamics, and evolving opportunities. These insights serve as the basis upon which businesses craft tailored approaches, optimize product offerings, and gain a competitive edge in an ever-growing marketplace.
The frequency of information updates is a cornerstone of our commitment to providing timely, relevant, and accurate insights. Cognizance Market Research adheres to a rigorous schedule of data collection, analysis, and distribution to ensure that our reports reflect the most current market realities. This proactive approach enables our clients to stay ahead of the curve, capitalize on emerging trends, and mitigate risks associated with outdated information.
Our research process is characterized by meticulous attention to detail and methodological rigor. It begins with a comprehensive understanding of client objectives, industry dynamics, and research scope. Leveraging a combination of primary and secondary research methodologies, we gather data from diverse sources including surveys, interviews, industry reports, and proprietary databases. Rigorous data analysis techniques are then employed to derive meaningful insights, identify patterns, and uncover actionable recommendations. Throughout the process, we remain vigilant in upholding the highest standards of data integrity, ensuring that our findings are robust, reliable, and actionable.
Key phases involved in in our research process are mentioned below:

Understanding Clients’ Objectives:
Extensive Discussions and Consultations:
Industry and Market Segment Analysis:
Target Audience Understanding:
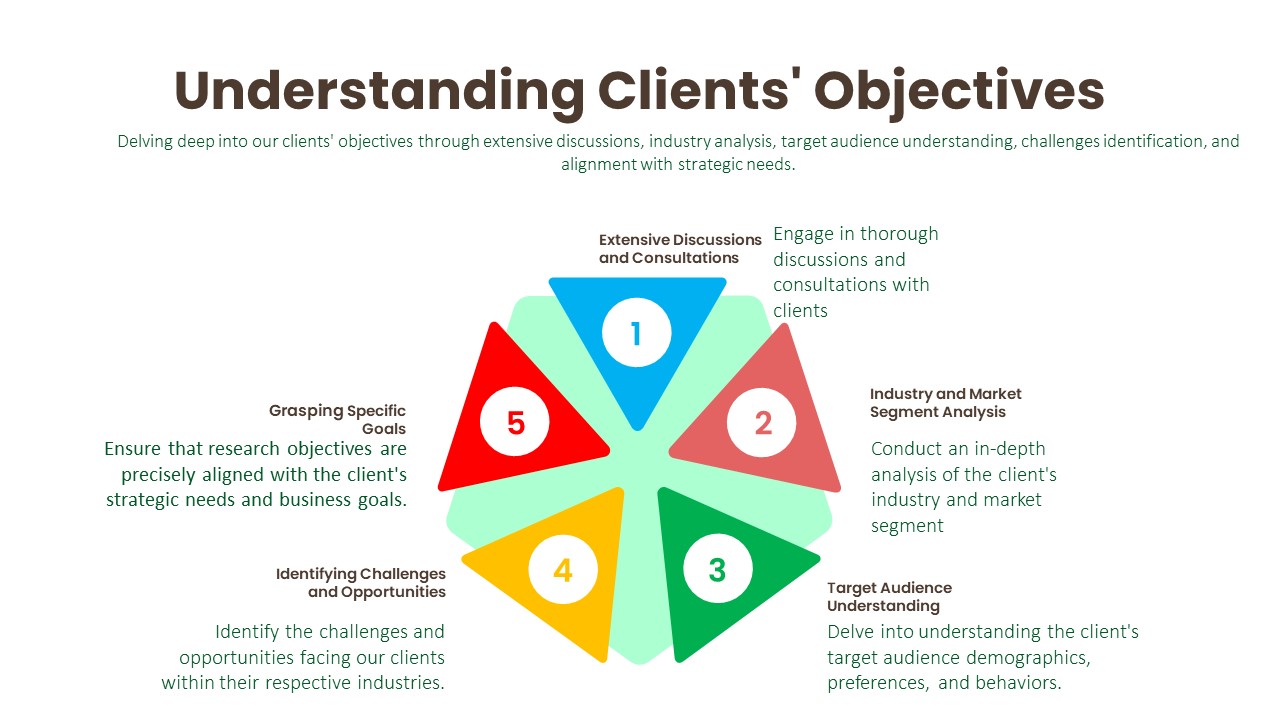
Identifying Challenges and Opportunities:
Grasping Specific Goals:
Data Collection:
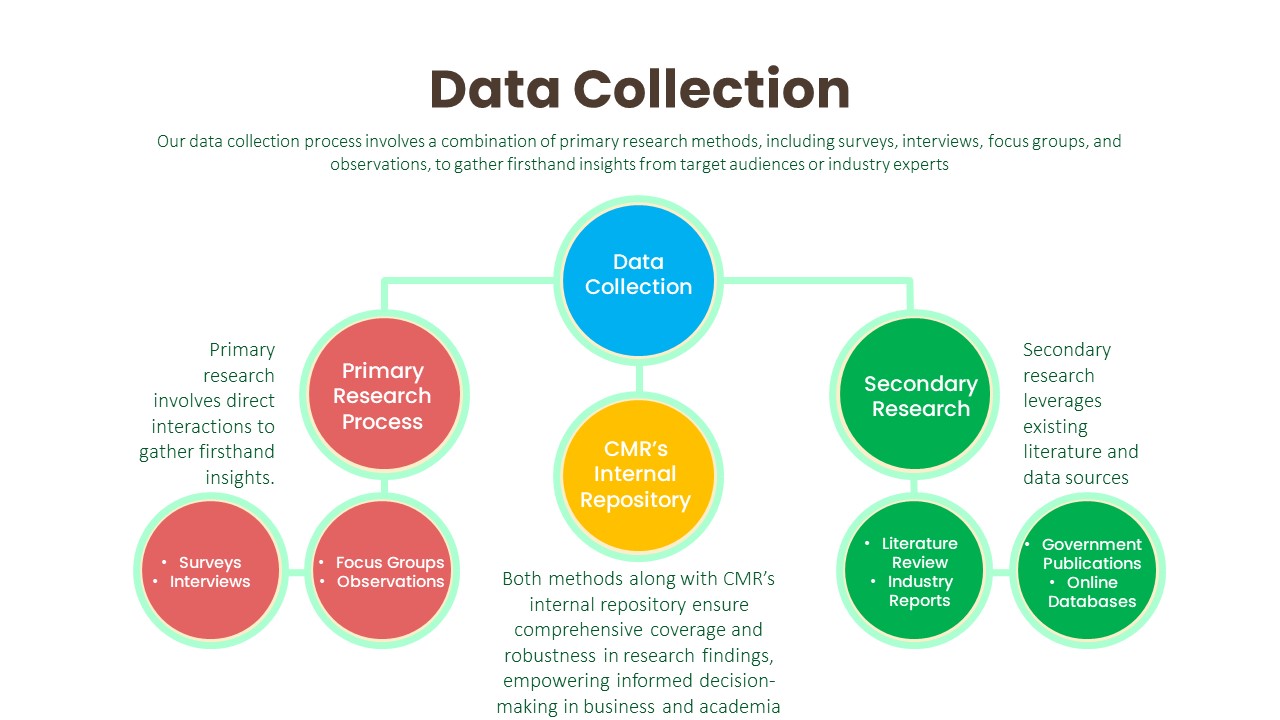
Primary Research Process:
Secondary Research Process:
Data Analysis:
The data analysis phase serves as a critical juncture where raw data is transformed into actionable insights that inform strategic decision-making. Through the utilization of analytical methods such as statistical analysis and qualitative techniques like thematic coding, we uncover patterns, correlations, and trends within the data. By ensuring the integrity and validity of our findings, we strive to provide clients with accurate and reliable insights that accurately reflect the realities of the market landscape.

Transformation of Raw Data:
Utilization of Analytical Methods:
Statistical Analysis:
Qualitative Analysis Techniques:
Integrity and Validity Maintenance:
Data Validation:
The final phase of our research methodology is data validation, which is essential for ensuring the reliability and credibility of our findings. Validation involves scrutinizing the collected data to identify any inconsistencies, errors, or biases that may have crept in during the research process. We employ various validation techniques, including cross-referencing data from multiple sources, conducting validity checks on survey instruments, and seeking feedback from independent experts or peer reviewers. Additionally, we leverage internal quality assurance protocols to verify the accuracy and integrity of our analysis. By subjecting our findings to rigorous validation procedures, we instill confidence in our clients that the insights they receive are robust, reliable, and trustworthy.
Importance of Data Validation:
Scrutiny of Collected Data:
Validation Techniques:
Internal Quality Assurance Protocols:
We can customize every report – free of charge – including purchasing stand-alone sections or country-level reports
We help clients to procure the report or sections of the report at their budgeted price. Kindly click on the below to avail

Cognizance market research is continuously guiding customers around the globe towards strategies for transformational growth. Today, businesses have to innovate more than ever before, not just to survive, but to succeed in the future

© 2023 All rights Reserved. Cognizance Market Research