
Global Cytomegalovirus Therapies Market – Overview
The term cytomegalovirus (CMV) therapies refer to a group of medical approaches used to treat infections brought on by the cytomegalovirus, a common herpesvirus that can significantly increase morbidity and mortality, especially in new-borns and immunocompromised people. With developments in antiviral medications, immune-based treatments, and preventive measures, the landscape of CMV infection treatment has changed dramatically over time. The many facets of CMV therapies, such as immunomodulatory treatments, antiviral medications, and preventative measures, will be covered in this overview. The primary component of CMV therapy is antiviral medication, which is intended to suppress viral growth, lower viral loads, and relieve CMV-related symptoms. First-line treatments for CMV infections, such as CMV retinitis, CMV colitis, and CMV pneumonitis, in immunocompromised patients—such as transplant recipients and HIV/AIDS patients—include ganciclovir and its oral prodrug valganciclovir. Alternative antiviral medications such as foscarnet and cidofovir are used to treat CMV infections, especially when ganciclovir-resistant forms of the virus exist or when ganciclovir therapy is no longer effective. Viral DNA polymerase is the focus of these antiviral medications, which prevent viral replication and reduce CMV replication in infected cells.
Immunotherapy-based treatments are being investigated as possible CMV infection treatments in addition to antiviral medications. By boosting or restoring cellular immunity, immunomodulatory treatments including adoptive transfer of CMV-specific T cells and CMV-specific T-cell therapy seek to improve the immune response against CMV. These treatments appear to be effective in lowering the risk of CMV-related problems and preventing CMV reactivation, especially in transplant patients and other immunocompromised people. In addition, studies have looked into passive immunization methods using monoclonal antibodies that target CMV glycoprotein B(gB) or phosphoprotein 65 (pp65) to stop CMV infection in high-risk groups such as transplant recipients and expectant mothers.
Global Cytomegalovirus Therapies Market – Competitive Insights
Nov 6, 2023 – Maribavir (Livecity) is a newer antiviral approved by the FDA for post-transplant patients with CMV infection refractory or resistant to existing treatments.
Sep 21, 2023 – Enrollment in a phase 1 trial of HIV vaccine that uses a cytomegalovirus (CMV) vector has started in the United States and South Africa, the National Institute of Allergy and Infectious Diseases (NIAID).
Some of the Key Players in the Global Cytomegalovirus Therapies Markets Include-
Global Cytomegalovirus Therapies Market – Growth Drivers
One of the primary growth drivers for the CMV therapies market is the increasing prevalence of CMV infection, particularly among immunocompromised individuals and neonates. CMV is a ubiquitous herpesvirus that infects people of all ages worldwide, with a higher prevalence observed in certain populations, such as transplant recipients, HIV/AIDS patients, and infants born to CMV-seropositive mothers. The potential severity of CMV-related complications, including organ rejection, graft-versus-host disease (GVHD), and congenital CMV infection, underscores the need for effective treatment options to prevent or mitigate adverse outcomes. The market for CMV therapeutics is expanding due to the growing need for potent antiviral treatments. Antiviral medications that prevent viral replication and lower viral load, such as cidofovir, foscarnet, ganciclovir, and valganciclovir, are frequently used to treat CMV infections. Drug resistance, treatment toxicity, and the scarcity of therapy alternatives for specific patient populations continue to be major issues, nevertheless. To address these issues and enhance treatment results for CMV-infected individuals, there is an increasing need for novel antiviral medicines, immune-based treatments, and preventative measures. The development of CMV diagnostics and treatment alternatives is also propelling market expansion. Advancements in diagnostic methodologies, like polymerase chain reaction (PCR) assays and antigenemia assays, facilitate prompt and precise identification of CMV infection, hence permitting prompt intervention and beginning of treatment. In order to strengthen the immune response to CMV and increase treatment efficacy, research efforts are also concentrated on creating innovative treatment modalities, such as immune-modulating medicines, antiviral monoclonal antibodies, and CMV-specific antiviral medications. The market for CMV therapeutics is expected to develop significantly due to the growing uses of CMV therapy in immunocompromised populations and transplant medicine. In transplant recipients and other immunocompromised individuals, CMV infection is a major source of morbidity and mortality. As such, effective prophylactic, preemptive medication, and treatment techniques are required to avoid or manage problems associated with CMV infection. It is anticipated that the use of CMV treatments in these patient groups would propel market expansion and encourage the creation of cutting-edge, patient-specific treatment modalities.
Global Cytomegalovirus Therapies Market – Restraints
The development of antiviral medication resistance in CMV-infected patients is a major barrier, especially for immunocompromised patients undergoing long-term antiviral therapy. Treatment efficacy and treatment failure can result from CMV’s tendency to evolve resistance mutations to routinely used antiviral medications, such as ganciclovir and its prodrug valganciclovir. Especially in high-risk patient populations, the emergence of drug-resistant strains of CMV presents issues for doctors in terms of choosing suitable treatment regimens and effectively managing CMV infections. One major barrier to the market for CMV therapeutics is the toxicity of the antiviral treatments now available for CMV infection. Antiviral medications used to treat CMV, such as ganciclovir and foscarnet, can have dose-dependent side effects that include nephrotoxicity, gastrointestinal problems, and myelosuppression (suppression of the bone marrow). These adverse effects, especially in susceptible patient populations with prior comorbidities or impaired renal function, may restrict the tolerance of antiviral therapy and require dose modifications or treatment cessation. The market for CMV medicines is constrained by the scarcity of efficient treatment alternatives for specific CMV-related side effects, such as CMV pneumonitis and retinitis. Antiviral medications are the mainstay of CMV treatment, although, in severe or resistant cases, they might not always be enough to manage CMV-related symptoms. Clinicians handling CMV-infected patients may find their options for therapy limited and their jobs made more difficult by the absence of adjuvant medicines or alternative treatment modalities for CMV-associated comorbidities.
Global Cytomegalovirus Therapies Market – Opportunities
The high incidence of CMV infection, especially in infants and immunocompromised people, presents one important opportunity. CMV is a widespread herpesvirus that affects people of all ages worldwide. However, some populations—such as transplant recipients, HIV/AIDS patients, and babies delivered to mothers who test positive for the virus—have a higher incidence than others. Effective treatment options are necessary to prevent or decrease poor outcomes due to the possible severity of CMV-related problems, such as organ rejection, GVHD, and neonatal CMV infection. Promising opportunities exist for the CMV treatments market due to advancements in antiviral medications. Antiviral medications that block viral replication, like cidofovir, foscarnet, ganciclovir, and valganciclovir, are frequently used to treat CMV infections. But these medications have the potential to cause side effects, drug resistance, and less-than-ideal results, especially in people with impaired immune systems. To address these issues and enhance treatment outcomes for CMV-infected patients, novel antiviral medicines, such as immune-modulating treatments, antiviral monoclonal antibodies, and CMV-specific antiviral medications, are being developed. Strategies for the prevention and treatment of congenital CMV infection in mothers and newborns can be developed as a result of the growing awareness of this serious public health issue. There are several ways to lessen the risk of congenital CMV infection in babies, including through maternal education, prenatal screening, and antiviral therapies during pregnancy.
Global Cytomegalovirus Therapies Market – Geographical Insights
The market for Cytomegalovirus Therapies is segmented into regions such as North America, Latin America, Europe, Asia-Pacific, the Middle East, and Africa. In Europe, congenitally infected babies, HIV/AIDS patients, and transplant recipients are among the susceptible groups most commonly affected by CMV infection, which is acknowledged as a common viral infection. European countries with advanced healthcare systems, such as the United Kingdom, Germany, and France, have established guidelines and protocols for the management of CMV infection in transplant recipients, including prophylactic and preemptive antiviral therapy strategies. CMV infection is common in North America and has a major impact on the morbidity and mortality of transplant recipients, immunocompromised patients, and congenitally infected newborns. With specialist clinics and research institutes devoted to the diagnosis and treatment of issues connected to CMV, the United States, and Canada boast strong healthcare infrastructures and experience in managing CMV infections. Although information on prevalence rates and treatment approaches is scarce in comparison to other regions, CMV infection is becoming more widely acknowledged in Latin America as a serious public health concern. Latin American nations have several obstacles, including restricted availability of medical services, testing centers, and antiviral drugs, which may affect the identification and treatment of CMV infection in susceptible groups.
Global Cytomegalovirus Therapies Market – Key Development
Aug 9, 2023 – FDA approved extending Prevymis use from 100 to 200 days post-transplant for CMV prophylaxis in adult HSCT recipients at risk for late CMV infection. The approval was based on a phase 3 study showing a significant reduction in clinically significant CMV infections with extended Prevymis use.
Research Methodology: Aspects
Market research is a crucial tool for organizations aiming to navigate the dynamic landscape of customer preferences, business trends, and competitive landscapes. At Cognizance Market Research, acknowledging the importance of robust research methodologies is vital to delivering actionable insights to our clientele. The significance of such methodologies lies in their capability to offer clarity in complexity, guiding strategic management with realistic evidence rather than speculation. Our clientele seek insights that excel superficial observations, reaching deep into the details of consumer behaviours, market dynamics, and evolving opportunities. These insights serve as the basis upon which businesses craft tailored approaches, optimize product offerings, and gain a competitive edge in an ever-growing marketplace.
The frequency of information updates is a cornerstone of our commitment to providing timely, relevant, and accurate insights. Cognizance Market Research adheres to a rigorous schedule of data collection, analysis, and distribution to ensure that our reports reflect the most current market realities. This proactive approach enables our clients to stay ahead of the curve, capitalize on emerging trends, and mitigate risks associated with outdated information.
Our research process is characterized by meticulous attention to detail and methodological rigor. It begins with a comprehensive understanding of client objectives, industry dynamics, and research scope. Leveraging a combination of primary and secondary research methodologies, we gather data from diverse sources including surveys, interviews, industry reports, and proprietary databases. Rigorous data analysis techniques are then employed to derive meaningful insights, identify patterns, and uncover actionable recommendations. Throughout the process, we remain vigilant in upholding the highest standards of data integrity, ensuring that our findings are robust, reliable, and actionable.
Key phases involved in in our research process are mentioned below:

Understanding Clients’ Objectives:
Extensive Discussions and Consultations:
Industry and Market Segment Analysis:
Target Audience Understanding:
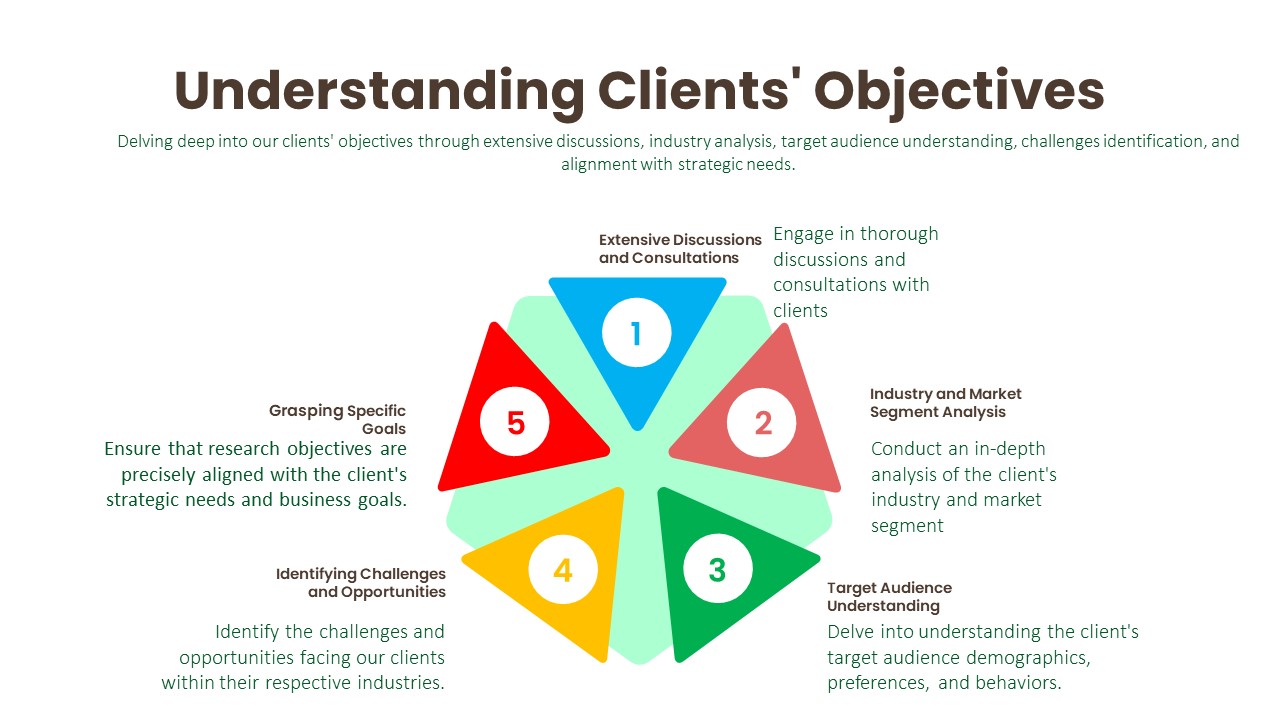
Identifying Challenges and Opportunities:
Grasping Specific Goals:
Data Collection:
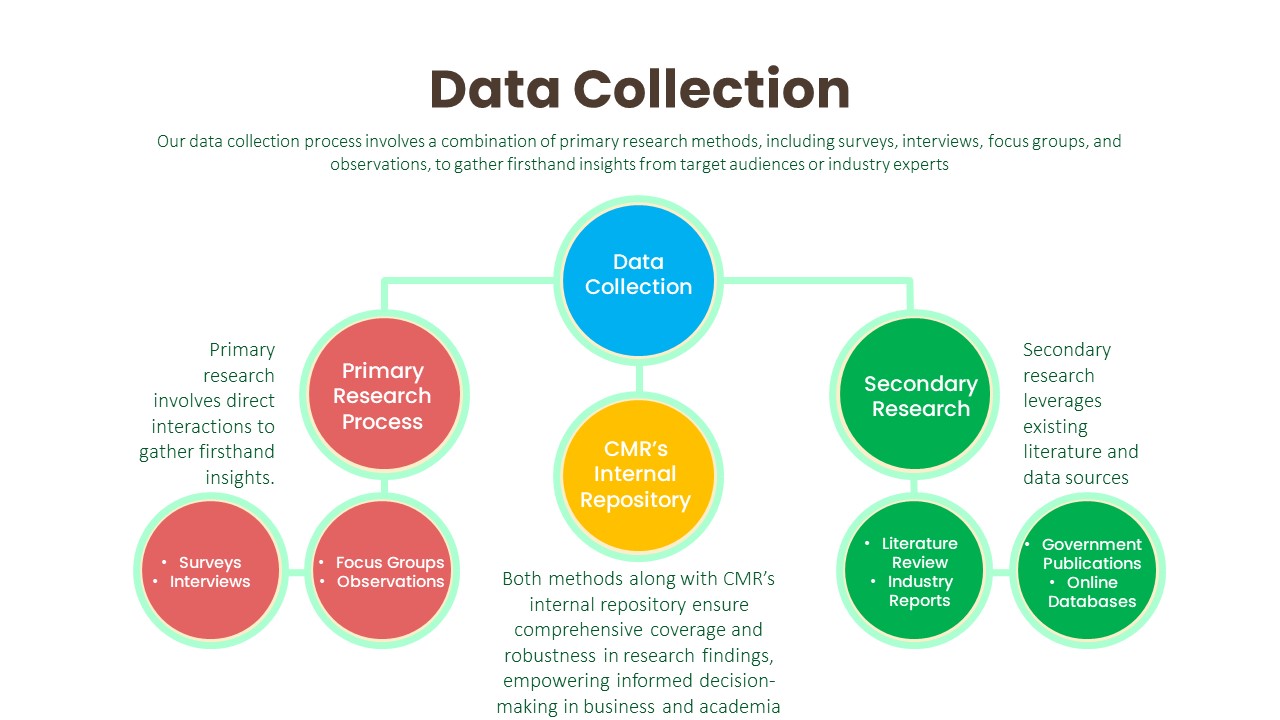
Primary Research Process:
Secondary Research Process:
Data Analysis:
The data analysis phase serves as a critical juncture where raw data is transformed into actionable insights that inform strategic decision-making. Through the utilization of analytical methods such as statistical analysis and qualitative techniques like thematic coding, we uncover patterns, correlations, and trends within the data. By ensuring the integrity and validity of our findings, we strive to provide clients with accurate and reliable insights that accurately reflect the realities of the market landscape.

Transformation of Raw Data:
Utilization of Analytical Methods:
Statistical Analysis:
Qualitative Analysis Techniques:
Integrity and Validity Maintenance:
Data Validation:
The final phase of our research methodology is data validation, which is essential for ensuring the reliability and credibility of our findings. Validation involves scrutinizing the collected data to identify any inconsistencies, errors, or biases that may have crept in during the research process. We employ various validation techniques, including cross-referencing data from multiple sources, conducting validity checks on survey instruments, and seeking feedback from independent experts or peer reviewers. Additionally, we leverage internal quality assurance protocols to verify the accuracy and integrity of our analysis. By subjecting our findings to rigorous validation procedures, we instill confidence in our clients that the insights they receive are robust, reliable, and trustworthy.
Importance of Data Validation:
Scrutiny of Collected Data:
Validation Techniques:
Internal Quality Assurance Protocols:
We can customize every report – free of charge – including purchasing stand-alone sections or country-level reports
We help clients to procure the report or sections of the report at their budgeted price. Kindly click on the below to avail

Cognizance market research is continuously guiding customers around the globe towards strategies for transformational growth. Today, businesses have to innovate more than ever before, not just to survive, but to succeed in the future

© 2023 All rights Reserved. Cognizance Market Research