Menu

Global Contract Research Organisation Services Market – Overview
The pharmaceutical, biotechnology, and medical device sectors rely heavily on Contract Research Organization (CRO) services because they offer a broad range of outsourced research and development (R&D) services. Preclinical research, clinical trials, regulatory submission, and post-market surveillance are just a few of the phases of the drug development process that these organizations can assist with their knowledge, infrastructure, and resources. Let’s take a closer look at CRO services, including their main features, functions, and importance in the life sciences industry. CRO services include a wide range of operations designed to make it easier to find, develop, and market novel medications, biologics, and medical devices. Pharmaceutical and biotech companies generally outsource these services to take advantage of the specialized knowledge, access to cutting-edge technology, and cost efficiencies offered by CROs. From early-phase research and preclinical studies to late-phase clinical trials and regulatory affairs support, the range of CRO services can be very different.
One of the primary functions of CROs is to conduct preclinical research and development activities, including in vitro and in vivo studies to evaluate the safety, efficacy, and pharmacokinetics of drug candidates. This may involve screening potential drug compounds, assessing their biological activity, and conducting toxicology studies to evaluate their safety profile. Preclinical research conducted by CROs helps pharmaceutical companies identify promising drug candidates and optimize their development pathways before advancing to clinical trials.
Another important function of CROs is clinical trial management, which includes organizing, carrying out, and supervising clinical trials to assess the security and effectiveness of experimental medications on human participants. In addition to working with sponsors to create clinical trial protocols, CROs also handle research participant recruitment, trial site management, data collection and analysis, and regulatory compliance. Sponsors can gain from expedited trial timeframes, streamlined operations, and access to specialist knowledge in therapeutic areas and study methodology by outsourcing clinical trial management to CROs. CROs offer a wide range of regulatory and quality assurance services to support sponsors in navigating complex regulatory landscapes and ensuring compliance with applicable regulations and guidelines. It also plays a crucial role in post-market surveillance and pharmacovigilance, monitoring the safety and effectiveness of marketed drugs and medical devices.
Global Contract Research Organisation Services Market- Competitive Insights
Apr 8, 2024 – Parexel, one of the world’s largest clinical research organisations (CROs) providing the full range of Phase I to IV clinical development services, today announced it has been named “Best Contract Research Organization” at the 17th Annual Vaccine Industry Excellence.
Mar 7, 2024 – Veeda Clinical Research, the Ahmedabad-based full-service clinical research organization (CRO) announced the appointment of Mahesh Bhalgat as the Group CEO.
Global Some of the Key Players in the global Contract Research Organisation Services Markets Include-
Global Contract Research Organisation Services Market- Growth Drivers
The growing complexity and expense of drug development is a major factor propelling the market for CRO services. In order to introduce novel medications to the market, pharmaceutical and biotechnology businesses must overcome a number of obstacles, such as strict regulatory requirements, protracted research schedules, and rising R&D expenses. Sponsors can take advantage of specialised knowledge, cost-effectiveness, and access to cutting-edge technology by outsourcing certain parts of drug development to CROs, which can expedite the process and shorten time-to-market. The demand for CRO services is also being driven by the globalisation of clinical trials. Pharmaceutical companies are expanding their clinical trial operations into more diverse geographic areas in an effort to reach a wider patient base, recruit patients more quickly, and handle the complexity of regulatory compliance. With their local knowledge, study site selection, and regulatory compliance, CROs are essential to the facilitation of international clinical trials. The need for CRO services is anticipated to increase as sponsors look to enter emerging regions and broaden their global presence. Additionally, the need for trained CRO services is being driven by the growing trend towards precision treatments and personalised medicine. Pharmaceutical companies are concentrating more on creating medicines unique to patient populations or disease subtypes as a result of advances in genetic sequencing, biomarker discovery, and targeted therapeutics. CROs that specialise in biomarker development, translational medicine, and genomics are in a good position to assist sponsors with the planning and execution of clinical trials related to personalised medicine projects. Lastly the increasing prevalence of chronic diseases, ageing populations, and the emergence of new therapeutic modalities such as biologics and cell and gene therapies are driving demand for CRO services.
Global Contract Research Organisation Services Market- Restraints
The market for CRO services is significantly constrained by the pressure on prices and growing competition. A growing number of CROs have been competing for contracts as the market for outsourced research and development services becomes more crowded. Because of the fierce competition, sponsors frequently try to negotiate cheaper service costs, which puts downward pressure on prices and profit margins. It is also becoming more difficult for established firms to stand out from the competition and hold onto market share as a result of the entry of new players into the industry, such as specialty and niche CROs. The pharmaceutical and biotechnology industries present considerable obstacles for CROs because of regulatory complications and compliance requirements. The process of developing new drugs is heavily regulated, with strict guidelines governing patient safety, data integrity, and regulatory filings. It can take a lot of time and resources for CROs to traverse complex regulatory frameworks and guarantee compliance with applicable laws and rules across numerous jurisdictions. Clinical research organizations and their sponsors may face consequences such as clinical trial delays, penalties, or suspension due to non-compliance with regulatory regulations. CROs deal with difficulties in attracting and keeping talent in a very competitive labor market. The proficiency and abilities of CRO personnel, which include project managers, regulatory affairs specialists, and clinical research specialists, are critical to the organization’s success. The business does, however, suffer from a lack of skilled workers with specific knowledge and expertise in fields like data analysis, regulatory affairs, and clinical trial management. The skill scarcity is further exacerbated by high turnover rates and rival talent poaching, making it challenging for CROs to attract and retain top people.
Contract Research Organisation Services Market- Opportunities
The growing intricacy and expense of drug development presents a major opportunity for the CRO services market. Companies in the pharmaceutical and biotechnology industries must contend with growing R&D expenses, onerous regulations, and protracted development schedules. Because of this, there is an increasing need for externalised research and development (R&D) services to expedite the drug development process, lower risks, and shorten time-to-market. Sponsors can more quickly and effectively introduce novel medications to patients thanks to CROs’ specialised knowledge, cost-saving measures, and access to cutting-edge technologies. The expansion of clinical trials worldwide offers CRO services a substantial opportunity. Pharmaceutical companies are expanding their clinical trial operations into more diverse geographic areas in an effort to reach a wider patient base, recruit patients more quickly, and handle the complexity of regulatory compliance. Due to their local knowledge, ability to set up research sites, and adherence to local laws, CROs are essential to the facilitation of international clinical trials. The need for CRO services is anticipated to increase as sponsors look to enter emerging regions and broaden their global presence. For CROs, the development of precision medicines and personalised medicine offers a substantial opportunity. Pharmaceutical companies are concentrating more on creating medicines unique to patient populations or disease subtypes as a result of advances in genetic sequencing, biomarker discovery, and targeted therapeutics. CROs that specialise in biomarker development, translational medicine, and genomics are in a good position to assist sponsors with the planning and execution of clinical trials related to personalised medicine projects. Complementary and alternative diagnostics (Comp) and patient stratification are two areas where CROs can profit from the increasing need for customised healthcare solutions.
Global Contract Research Organisation Services Market- Geographical Insights
The market for Contract Research Organisation Services is segmented into regions such as North America, Latin America, Europe, Asia-Pacific, the Middle East, and Africa. The pharmaceutical and biotechnology industries, well-established regulatory frameworks, and a high degree of research and development (R&D) activity have made North America—particularly the United States—the largest global market for CRO services. The area gains from having access to cutting-edge technologies, a sizable pool of skilled clinical research experts, and state-of-the-art research facilities. Europe is a big market for CRO services as well since important centers of clinical research and drug development are found there, including the UK, Germany, and France. The area benefits from having access to an extensive diversity of patient groups, a strong regulatory framework, and an established clinical trial infrastructure. Due to their sizable and varied patient populations, hospitable regulatory frameworks, and more affordable pricing when compared to North America and Europe, Latin American nations like Brazil, Mexico, and Argentina are drawing clinical trials. As a result, the region is becoming an increasingly important market for CRO services. Because of its closeness to the US, North American pharmaceutical companies looking to perform trials in Latin America can work together and outsource these tasks to the area.
Global Contract Research Organisation Services Market- Key Development
Feb 16, 2024 – Pharma and biotechs prefer midsize CROs over large CROs due to personalized service and concerns about the ‘one-stop shop’ model. Midsize CROs are favored for their agility, personalized attention, and access to senior-level expertise. A shift from large to midsize CROs is expected in the next three years, supported by industry reports.
Research Methodology: Aspects
Market research is a crucial tool for organizations aiming to navigate the dynamic landscape of customer preferences, business trends, and competitive landscapes. At Cognizance Market Research, acknowledging the importance of robust research methodologies is vital to delivering actionable insights to our clientele. The significance of such methodologies lies in their capability to offer clarity in complexity, guiding strategic management with realistic evidence rather than speculation. Our clientele seek insights that excel superficial observations, reaching deep into the details of consumer behaviours, market dynamics, and evolving opportunities. These insights serve as the basis upon which businesses craft tailored approaches, optimize product offerings, and gain a competitive edge in an ever-growing marketplace.
The frequency of information updates is a cornerstone of our commitment to providing timely, relevant, and accurate insights. Cognizance Market Research adheres to a rigorous schedule of data collection, analysis, and distribution to ensure that our reports reflect the most current market realities. This proactive approach enables our clients to stay ahead of the curve, capitalize on emerging trends, and mitigate risks associated with outdated information.
Our research process is characterized by meticulous attention to detail and methodological rigor. It begins with a comprehensive understanding of client objectives, industry dynamics, and research scope. Leveraging a combination of primary and secondary research methodologies, we gather data from diverse sources including surveys, interviews, industry reports, and proprietary databases. Rigorous data analysis techniques are then employed to derive meaningful insights, identify patterns, and uncover actionable recommendations. Throughout the process, we remain vigilant in upholding the highest standards of data integrity, ensuring that our findings are robust, reliable, and actionable.
Key phases involved in in our research process are mentioned below:

Understanding Clients’ Objectives:
Extensive Discussions and Consultations:
Industry and Market Segment Analysis:
Target Audience Understanding:
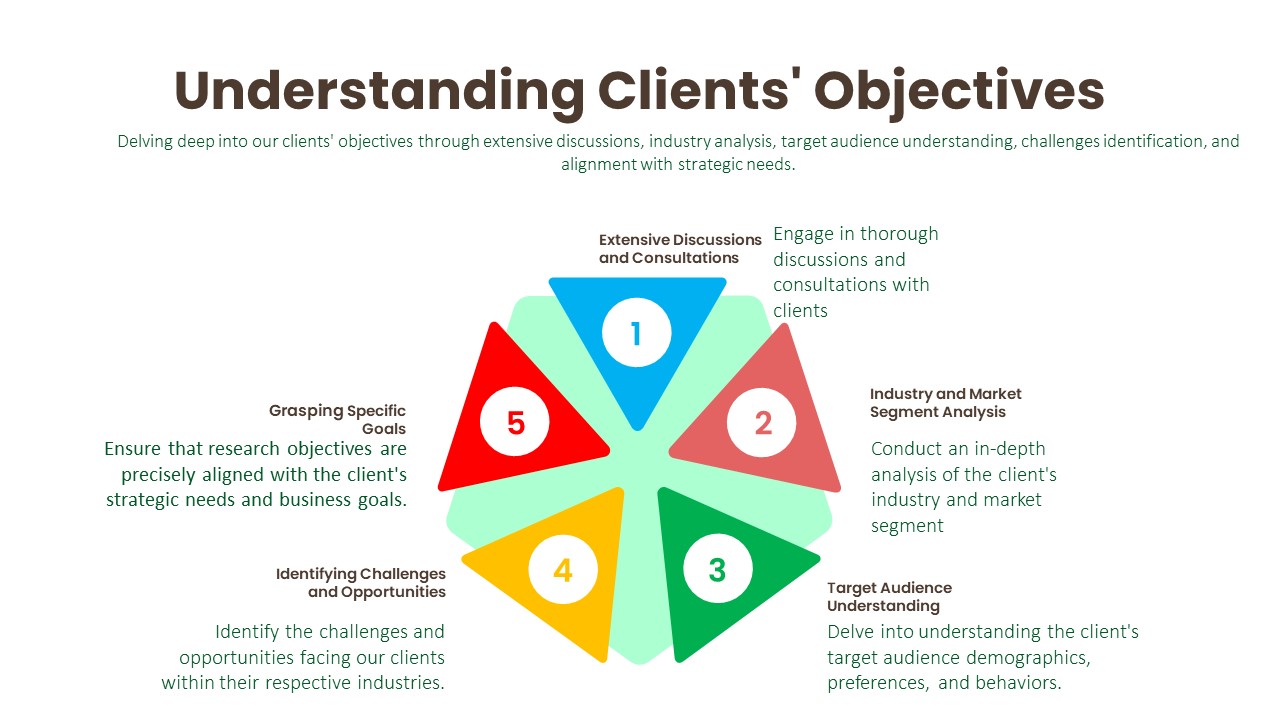
Identifying Challenges and Opportunities:
Grasping Specific Goals:
Data Collection:
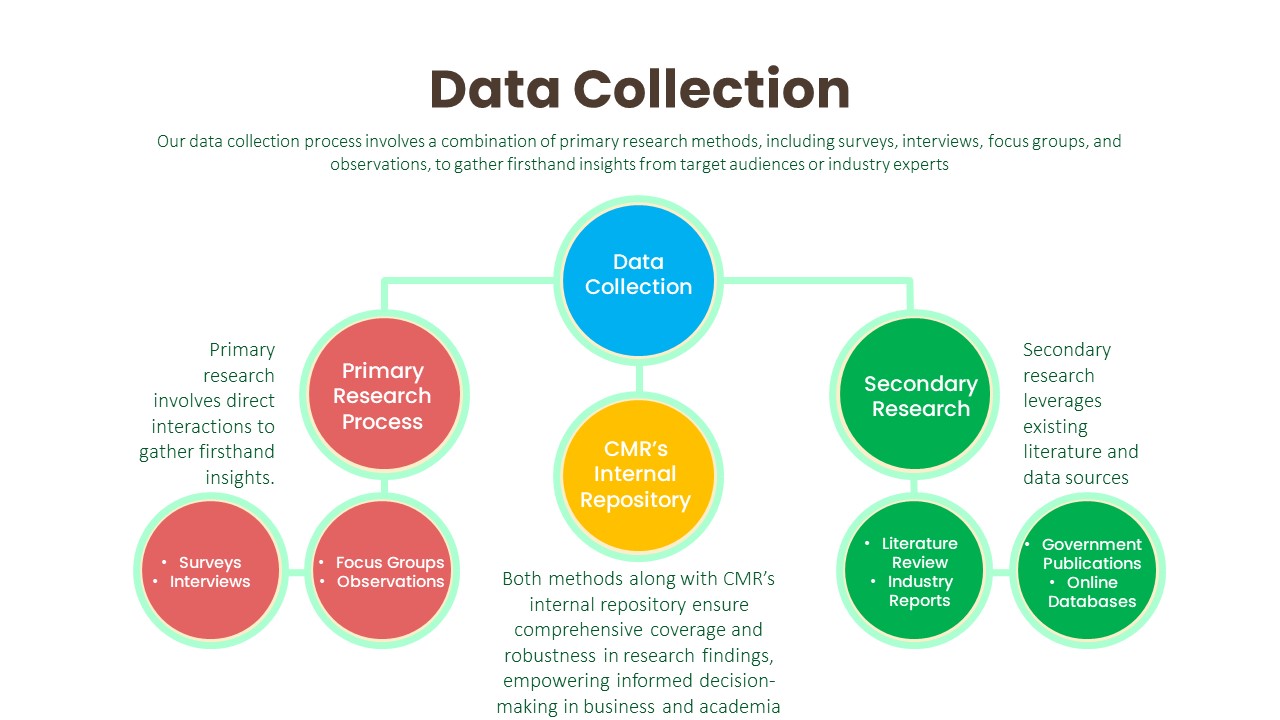
Primary Research Process:
Secondary Research Process:
Data Analysis:
The data analysis phase serves as a critical juncture where raw data is transformed into actionable insights that inform strategic decision-making. Through the utilization of analytical methods such as statistical analysis and qualitative techniques like thematic coding, we uncover patterns, correlations, and trends within the data. By ensuring the integrity and validity of our findings, we strive to provide clients with accurate and reliable insights that accurately reflect the realities of the market landscape.

Transformation of Raw Data:
Utilization of Analytical Methods:
Statistical Analysis:
Qualitative Analysis Techniques:
Integrity and Validity Maintenance:
Data Validation:
The final phase of our research methodology is data validation, which is essential for ensuring the reliability and credibility of our findings. Validation involves scrutinizing the collected data to identify any inconsistencies, errors, or biases that may have crept in during the research process. We employ various validation techniques, including cross-referencing data from multiple sources, conducting validity checks on survey instruments, and seeking feedback from independent experts or peer reviewers. Additionally, we leverage internal quality assurance protocols to verify the accuracy and integrity of our analysis. By subjecting our findings to rigorous validation procedures, we instill confidence in our clients that the insights they receive are robust, reliable, and trustworthy.
Importance of Data Validation:
Scrutiny of Collected Data:
Validation Techniques:
Internal Quality Assurance Protocols:
We can customize every report – free of charge – including purchasing stand-alone sections or country-level reports
We help clients to procure the report or sections of the report at their budgeted price. Kindly click on the below to avail

Cognizance market research is continuously guiding customers around the globe towards strategies for transformational growth. Today, businesses have to innovate more than ever before, not just to survive, but to succeed in the future

© 2023 All rights Reserved. Cognizance Market Research