
Clinical Trial Imaging Market - Global Industry, Analysis, Size, Share, Growth, Trends, and Forecasts 2023-2030
- Category: Healthcare
- Report ID: CMR15705
- Upcoming
Clinical trial imaging is a research study conducted with people who volunteer to take part. Clinical trials include the discovery, preclinical and clinical testing, regulatory filing, and life cycle management of a drug product. The clinical phase is subdivided into 3 phases phase I, phase II, and phase III, which form the basis of progressing a drug candidate through development towards regulatory submission. Before the start of clinical trials, a preclinical trial takes place in which the primary objective is to establish the safety profile of the drug candidate and establish the pharmacokinetic and pharmacodynamic properties of the compound. Phase 1 clinical trial is the first opportunity to evaluate the drug candidate’s clinical evaluation of safety and tolerability through dose range. In phase 1 clinical trials in which subjects typically have refractory advanced solid tumors, imaging is routinely applied to either acquire any evidence of anti-tumor activity via lesion shrinkage on CT or interrogate pharmacodynamics that may relate to the drug action. Once an acceptable safety profile has been established and the drug dose and schedule defined, a phase II trial can be planned. This trial aims to evaluate drug efficacy and safety within a targeted patient population. It established the first opportunity for a robust efficacy evaluation for an intended indication. In phase II clinical imaging is typically used and assessed using standard response criteria such as RECIST. Phase III trials aim to provide substantive evidence regarding the safety and efficacy of a drug within a large population for which drug administration is indicated. Most phase III trials in solid tumors utilize established RECIST criteria based on established and routine imaging methods.
Imaging is utilized on a day-to-day basis for evaluating disease response, the context in which imaging is used in clinical trials differs to some degree from how this is utilized in clinical trials. Functional imaging techniques such as PET/CT, CT perfusion imaging, DCE-MRI, DW-MRI, and MRS have been utilized to form drug effects in clinical trials and have also sometimes provided unique prognostic information.
Global Clinical Trial Imaging Market – Competitive Landscape
On November 27, 2023, Aidoc Unveils USD30 million investment in a revolutionary foundation model for imaging AI at RSNA 2023. On November 27, 2023, Cleveland Clinic and Canon Inc. entered a partnership to advance global innovation medical imaging solutions. On October 10, 2023, Japanese firm Mitsui invested USD 8 million in US-based clinical intelligence startup Lokavant. On July 13, 2023, NVIDIA invested USD 50 million in AI-enabled drug discovery. On March 8, 2023, Lilly provided an update on the A4 study of Solanezumab for preclinical Alzheimer’s Disease. On December 23, 2022, CapVest’s GLO Healthcare completes the acquisition of Calyx, a global leader in the delivery of improved outcomes from clinical trials.
Some of the Key Players in the Global Clinical Trial Imaging Market Include –
- Icon PLC
- Wirb-Copernicus Group
- Ixico PLC
- Navitas Clinical Research, Inc
- Bio Telemetry Inc.
- Biomedical System Corporation
- Medpace
- WCG Clinical
Global Clinical Trial Imaging Market – Growth Drivers
The rising prevalence of chronic diseases, including cancer and cardiovascular disorders, drives the demand for clinical trials utilizing advanced imaging technologies. Imaging plays a crucial role in disease diagnosis, monitoring, and treatment evaluation. According to World Health Organization non-communicable diseases such as chronic disease kills USD 41 million people each year, equivalent to 74% of all death globally. Continuous innovation in imaging modalities such as MRI, CT Scan, and PET Scan contributes to the growth of the clinical trial imaging market. Enhanced precision, resolution, and speed of imaging techniques attract pharmaceutical companies and researchers. The growing emphasis on precision medicine, tailoring treatments based on individual characteristics, boosts the demand for imaging studies. Imaging biomarkers and personalized diagnostic approaches play a key role in developing targeted therapies. The globalization of clinical trials, with an increasing number conducted in diverse geographic regions, fuels the demand for standardized and advanced imaging services. This trend opens new opportunities for market expansion and collaboration with international stakeholders. The widespread involvement of contract research organizations in clinical trials leads to increased demand for imaging, driving efficiency and supporting pharmaceutical companies in their research endeavors.
Global Clinical Trial Imaging Market – Restraints
Adopting advanced imaging technologies in clinical trials involves substantial upfront costs for equipment, software, and staff training. This financial barrier poses a restraint, particularly for smaller pharmaceutical companies and research organizations with limited budgets. According to ASPE GOV highest clinical research burden across all phases is the respiratory system USD 115.3 million followed by pain and anesthesia USD 105.4 million and oncology USD 78.6 million. The complex and varied regulatory landscape for clinical trial imaging across different regions poses a challenge. Adhering to diverse regulatory standards requires meticulous planning and execution, potentially slowing down the initiation and progress of global clinical trials. The increasing reliance on digital imaging and the collection of sensitive patient data raise concerns about data security and privacy. Stricter regulations and the need for secure data management solutions add complexities and potential hurdles to the clinical trial imaging market. The lack of standardized imaging protocols across different therapeutic areas and regions hampers the comparability and consistency of imaging data. This elimination complicates multi-center trials and may affect the reliability of results. The operations and analysis of advanced imaging technologies require specialized skills. A shortage of skilled personnel in the field of clinical trial imaging poses a restraint, leading to potential delays and increased competition for qualified professionals.
Global Clinical Trial Imaging Market – Opportunities
The integration of artificial intelligence and machine learning enhances the precision and efficiency of image analysis, contributing to more robust and reliable clinical trial results. The shift towards decentralized and remote clinical trials creates opportunities for imaging services. Remote patient monitoring and imaging data collection offer cost-effective and patient-centric alternatives, especially in post COVID-19 landscape. The growing importance of data analytics and integration in clinical trials presents opportunities for imaging market players. Efficient data management, analysis, and integration of imaging data with other trial endpoints contribute to more comprehensive and insightful outcomes.
Global Clinical Trial Imaging Market – Geographical Insight
The market for global clinical trial imaging is segmented into regions such as North America, Latin America, Europe, Asia-Pacific, the Middle East & Africa. Asia-Pacific is a rapidly growing market for clinical trial imaging due to a rapid increase in clinical trial activities, driven by factors such as a large and diverse patient population, lower operational costs, and supportive regulatory frameworks in countries like China and India. European countries collaborate through imaging networks, fostering knowledge exchange and standardization. This collaboration enhances the efficiency and reliability of clinical trial imaging data across borders. North America, particularly the United States, holds a leading position in global clinical research. The region’s well-established research infrastructure and regulatory framework make it a hub for clinical trials including those utilizing advanced imaging technologies.
Global Clinical Trial Imaging Market – Key Development
- On October 10, 2023, UTA created a USD 6.2 million clinical imaging research center.
- On October 5, 2023, Imperial partners in the UK launched the first total-body PET platform for drug discovery.
- On July 24, 2023, PathAI launched AIM-HER2 breast cancer, an artificial intelligence-powered HER2 scoring algorithm for Biopharma Research and Clinical Labs.
- On June 9, 2023, Serac Imaging launched clinical testing of a portable gamma camera in the US.
- On March 13, 2023, Clario launched a Cloud-based image viewer for clinical trials USA.
Research Methodology: Aspects
Market research is a crucial tool for organizations aiming to navigate the dynamic landscape of customer preferences, business trends, and competitive landscapes. At Cognizance Market Research, acknowledging the importance of robust research methodologies is vital to delivering actionable insights to our clientele. The significance of such methodologies lies in their capability to offer clarity in complexity, guiding strategic management with realistic evidence rather than speculation. Our clientele seek insights that excel superficial observations, reaching deep into the details of consumer behaviours, market dynamics, and evolving opportunities. These insights serve as the basis upon which businesses craft tailored approaches, optimize product offerings, and gain a competitive edge in an ever-growing marketplace.
The frequency of information updates is a cornerstone of our commitment to providing timely, relevant, and accurate insights. Cognizance Market Research adheres to a rigorous schedule of data collection, analysis, and distribution to ensure that our reports reflect the most current market realities. This proactive approach enables our clients to stay ahead of the curve, capitalize on emerging trends, and mitigate risks associated with outdated information.
Our research process is characterized by meticulous attention to detail and methodological rigor. It begins with a comprehensive understanding of client objectives, industry dynamics, and research scope. Leveraging a combination of primary and secondary research methodologies, we gather data from diverse sources including surveys, interviews, industry reports, and proprietary databases. Rigorous data analysis techniques are then employed to derive meaningful insights, identify patterns, and uncover actionable recommendations. Throughout the process, we remain vigilant in upholding the highest standards of data integrity, ensuring that our findings are robust, reliable, and actionable.
Key phases involved in in our research process are mentioned below:

Understanding Clients’ Objectives:
Extensive Discussions and Consultations:
- We initiate in-depth discussions and consultations with our clients to gain a comprehensive understanding of their objectives. This involves actively listening to their needs, concerns, and aspirations regarding the research project.
- Through these interactions, we aim to uncover the underlying motivations driving their research requirements and the specific outcomes they hope to achieve.
Industry and Market Segment Analysis:
- We invest time and effort in comprehensively understanding our clients’ industry and market segment. This involves conducting thorough research into market trends, competitive dynamics, regulatory frameworks, and emerging opportunities or threats.
- By acquiring a deep understanding of the broader industry landscape, we can provide context-rich insights that resonate with our clients’ strategic objectives.
Target Audience Understanding:
- We analyze our clients’ target audience demographics, behaviors, preferences, and needs to align our research efforts with their consumer-centric objectives. This entails segmenting the audience based on various criteria such as age, gender, income level, geographic location, and psychographic factors.
- By understanding the nuances of the target audience, we can tailor our research methodologies to gather relevant data that illuminates consumer perceptions, attitudes, and purchase intent.
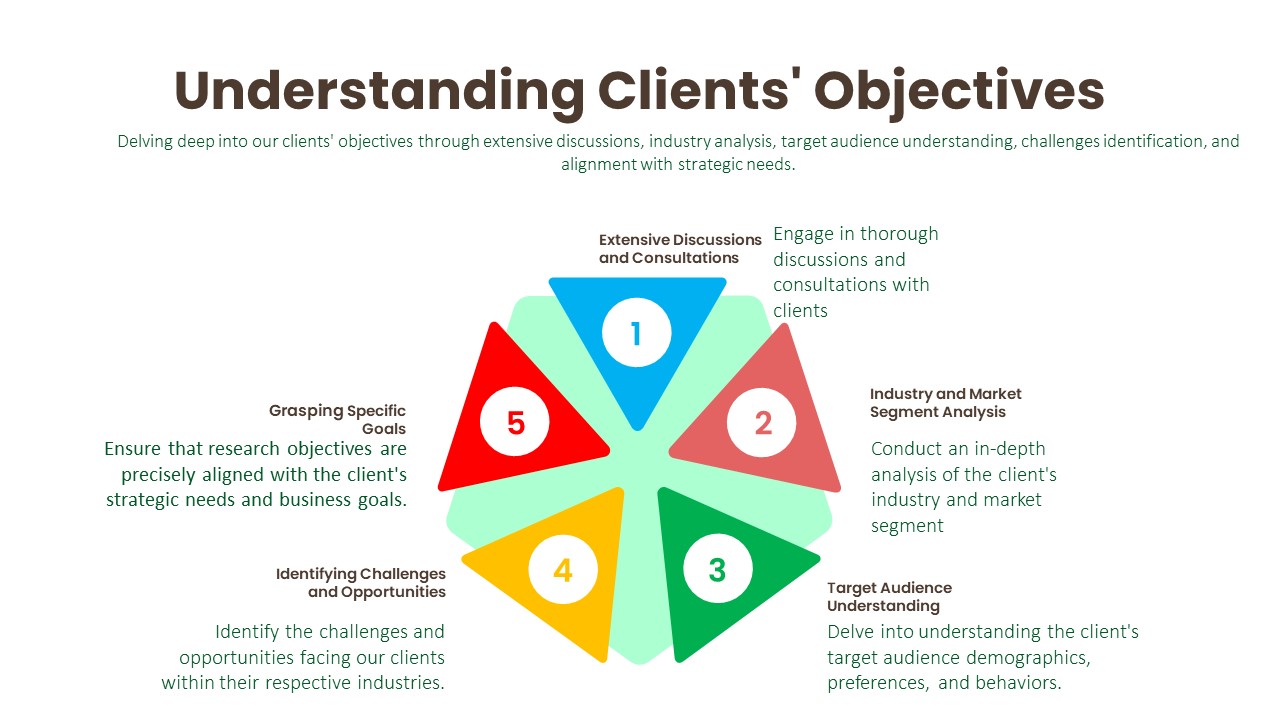
Identifying Challenges and Opportunities:
- We proactively identify the challenges and opportunities facing our clients within their respective industries. This involves conducting SWOT (Strengths, Weaknesses, Opportunities, Threats) analyses and competitive benchmarking exercises.
- By identifying potential obstacles and growth drivers, we can provide strategic recommendations that help our clients navigate complexities and capitalize on emerging opportunities effectively.
Grasping Specific Goals:
- We delve into the intricacies of our clients’ objectives to gain clarity on the specific goals they aim to accomplish through the research. This entails understanding their desired outcomes, such as market expansion, product development, or competitive analysis.
- By gaining a nuanced understanding of our clients’ goals, we can tailor our research approach to address their unique challenges and opportunities effectively.
Data Collection:
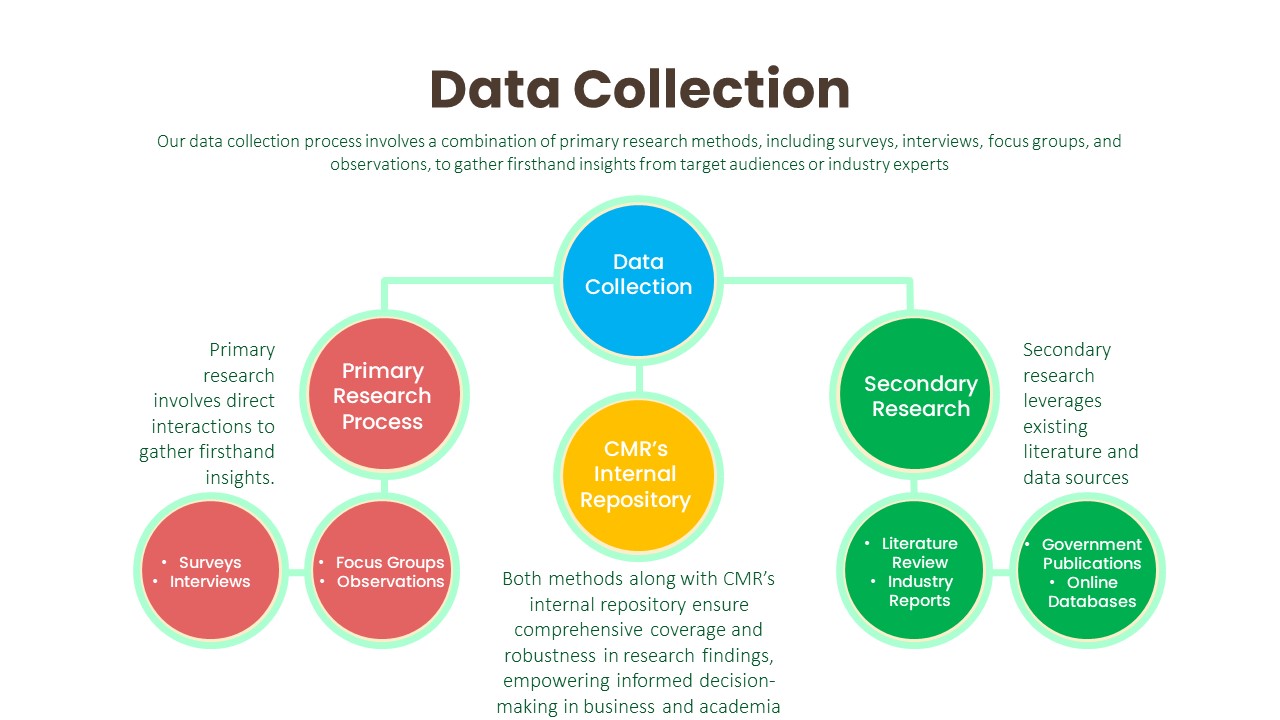
Primary Research Process:
- Surveys: We design and administer surveys tailored to capture specific information relevant to our clients’ objectives. This may involve employing various survey methodologies, such as online, telephone, or face-to-face interviews, to reach target audiences effectively.
- Interviews: We conduct structured or semi-structured interviews with key stakeholders, industry experts, or target consumers to gather in-depth insights and perspectives on relevant topics. These interviews allow us to probe deeper into specific issues and uncover valuable qualitative data.
- Focus Groups: We organize focus group discussions with carefully selected participants to facilitate interactive discussions and gather collective opinions, attitudes, and preferences. This qualitative research method provides rich contextual insights into consumer behaviors and perceptions.
- Observations: We conduct observational research by directly observing consumer behaviors, interactions, and experiences in real-world settings. This method enables us to gather objective data on consumer actions and reactions without relying on self-reported information.
Secondary Research Process:
- Literature Review: We conduct comprehensive literature reviews to identify existing studies, academic articles, and industry reports relevant to the research topic. This helps us gain insights into previous research findings, theoretical frameworks, and best practices.
- Industry Reports: We analyze industry reports published by reputable trade associations (whitepapers, research studies, etc.), and government agencies (U.S. Census Bureau, Bureau of Labor Statistics, and Securities and Exchange Commission etc.) to obtain macro-level insights into market trends, competitive landscapes, and industry dynamics.
- Government Publications: We review government publications, such as economic reports, regulatory documents, and statistical databases, to gather relevant data on demographics, market size, consumer spending patterns, and regulatory frameworks.
- Online Databases: We leverage online databases, such as industry portals, and academic repositories (PubMed Central (PMC), ScienceDirect, SSRN (Social Science Research Network), Directory of Open Access Journals (DOAJ), NCBI, etc.), to access a wide range of secondary data sources, including market statistics, financial data, and industry analyses.
Data Analysis:
The data analysis phase serves as a critical juncture where raw data is transformed into actionable insights that inform strategic decision-making. Through the utilization of analytical methods such as statistical analysis and qualitative techniques like thematic coding, we uncover patterns, correlations, and trends within the data. By ensuring the integrity and validity of our findings, we strive to provide clients with accurate and reliable insights that accurately reflect the realities of the market landscape.

Transformation of Raw Data:
- Upon collecting the necessary data, we transition into the data analysis phase, where raw data is processed and transformed into actionable insights. This involves organizing, cleaning, and structuring the data to prepare it for analysis.
Utilization of Analytical Methods:
- Depending on the research objectives, we employ a diverse range of analytical methods to extract meaningful insights from the data. These methods include statistical analysis, trend analysis, regression analysis, and qualitative coding.
Statistical Analysis:
- Statistical tools are instrumental in uncovering patterns, correlations, and trends within the data. By applying statistical techniques such as descriptive statistics, hypothesis testing, and multivariate analysis, we can discern relationships and derive valuable insights.
Qualitative Analysis Techniques:
- In addition to quantitative analysis, we leverage qualitative analysis techniques to gain deeper insights from qualitative data sources such as interviews or open-ended survey responses. One such technique is thematic coding, which involves systematically categorizing and interpreting themes or patterns within qualitative data.
Integrity and Validity Maintenance:
- Throughout the analysis process, we maintain a steadfast commitment to upholding the integrity and validity of our findings. This entails rigorous adherence to established methodologies, transparency in data handling, and thorough validation of analytical outcomes.
Data Validation:
The final phase of our research methodology is data validation, which is essential for ensuring the reliability and credibility of our findings. Validation involves scrutinizing the collected data to identify any inconsistencies, errors, or biases that may have crept in during the research process. We employ various validation techniques, including cross-referencing data from multiple sources, conducting validity checks on survey instruments, and seeking feedback from independent experts or peer reviewers. Additionally, we leverage internal quality assurance protocols to verify the accuracy and integrity of our analysis. By subjecting our findings to rigorous validation procedures, we instill confidence in our clients that the insights they receive are robust, reliable, and trustworthy.
Importance of Data Validation:
- Data validation is the final phase of the research methodology, crucial for ensuring the reliability and credibility of the findings. It involves a systematic process of reviewing and verifying the collected data to detect any inconsistencies, errors, or biases.
Scrutiny of Collected Data:
- The validation process begins with a thorough scrutiny of the collected data to identify any discrepancies or anomalies. This entails comparing data points, checking for outliers, and verifying the accuracy of data entries against the original sources.
Validation Techniques:
- Various validation techniques are employed to ensure the accuracy and integrity of the data. These include cross-referencing data from multiple sources to corroborate findings, conducting validity checks on survey instruments to assess the reliability of responses, and seeking feedback from independent experts or peer reviewers to validate the interpretation of results.
Internal Quality Assurance Protocols:
- In addition to external validation measures, internal quality assurance protocols are implemented to further validate the accuracy of the analysis. This may involve conducting internal audits, peer reviews, or data validation checks to ensure that the research process adheres to established standards and guidelines.
We can customize every report – free of charge – including purchasing stand-alone sections or country-level reports
We help clients to procure the report or sections of the report at their budgeted price. Kindly click on the below to avail



