
Classical Homocystinuria and HCU is an inherited disorder of the metabolism of the amino acid methionine due to a deficiency of cystathionine beta-synthase or methionine synthase. It is an inherited autosomal recessive trait, which means a child needs to inherit a copy of the defective gene from both parents to be affected. Symptoms of homocystinuria can also be caused by a deficiency of vitamins B6, B12, or folate. This defect leads to a multi-systemic disorder of the connective tissue, muscles, central nervous system, and cardiovascular system. Homocystinuria represents a group of hereditary metabolic disorders characterized by an accumulation of the amino acid homocysteine in the serum and an increased excretion of homocysteine in the urine. Infants appear to be normal and early symptoms, if any are vague. Signs and symptoms of homocystinuria may include a family history of homocystinuria, flush across the cheeks, musculoskeletal, intellectual disability, seizures, psychiatric disease, eye anomalies, and vascular disease. It is usually caused by the deficiency of the enzyme cystathionine beta-synthase, mutations of other related enzymes such as methionine synthase, or the deficiency of folic acid, vitamin B12, and/or pyridoxine. The term homocystinuria describes an increased excretion of the thiol amino acid homocysteine in urine. The source of this increase may be one of which is CBS deficiency. No specific cure has been discovered for homocystinuria, however many people are treated using high doses of vitamin B6. Slightly less than 50% respond to this treatment and need to take supplemental vitamin B6 for the rest of their lives.
Different organizations, companies, and physicians continuously try to find innovative treatments and medications to treat patients. Companies testing new drugs in clinical trials to launch innovative drugs into the market.
Global Classical Homocystinuria Market – Competitive Landscape
On June 22, 2023, Aeglea BioTherapeutics acquired Spyre Therapeutics and Received USD 210 million in private investment to advance inflammatory treatments. Companies involved in the development and marketing of treatments for classical homocystinuria may include pharmaceutical and biotechnology firms.
Some of the Key Players in the Global Classical Homocystinuria Market Include –
Global Classical Homocystinuria Market – Growth Drivers
The rising number of reported classical homocystinuria cases globally contributes to the expansion of the market of classical homocystinuria, creating a demand for effective treatments. According to the National Center for Biotechnology Information (NCBI), the prevalence of classical homocystinuria is approximately 1 in 200,000 – 335,000 worldwide. Ongoing progress in genetic research facilitates a deeper understanding of classical homocystinuria, paving the way for the development of targeted and personalized treatment approaches. The exploration and development of innovative therapeutic strategies, including gene therapies and enzyme replacement therapies, drive growth by offering novel and potentially more effective treatment options. Supportive regulatory frameworks, orphan drug designations, and government incentives for rare disease treatments create a conducive environment for companies to invest in classical homocystinuria research and development. Collaborative efforts between pharmaceutical companies, research institutions, and advocacy groups accelerate the development and commercialization of classical homocystinuria therapies, fostering a dynamic and cooperative ecosystem. Increasing awareness and recognition of the importance of addressing rare diseases leads to greater investments, attracting funding and resources for classical homocystinuria research and development.
Global Classical Homocystinuria Market – Restraints
The rare nature of classical homocystinuria results in a relatively small patient population, posing a challenge to the scale and widespread market penetration of classical homocystinuria. Research and development costs for rare disease treatments can be prohibitively high, impacting the financial viability of potential therapies and hindering investment in this niche market of classical homocystinuria market. According to the Orphanet Journal of Rare Diseases clinical costs per approved orphan drug are USD 166 million. Orphan drug designation notwithstanding, navigating complex regulatory pathways for rare diseases can lead to delays in product approvals, adding to the time and costs associated with bringing treatments to market for classical homocystinuria. Limited awareness of classical homocystinuria among healthcare professionals may lead to delayed misdiagnoses, affecting the timely initiation of appropriate treatments. Patients may face challenges in accessing costly treatments, and reimbursement hurdles can limit affordability, impacting the widespread adoption of therapies for classical homocystinuria. Classical homocystinuria exhibits genetic heterogeneity, making it challenging to develop universal treatment approaches that effectively address the diverse genetic mutations associated with the condition. Once treatments gain market approval, the potential entry of generic alternatives can lead to price erosion and increased competition, affecting the revenue potential for original therapies. Despite increasing awareness, classical homocystinuria may receive less research funding compared to more prevalent diseases, limiting the resources available for developing and improving treatment options.
Global Classical Homocystinuria Market – Opportunities
The increasing prevalence of classical homocystinuria presents a significant market opportunity, driven by a rising number of diagnosed cases globally. Ongoing research and development efforts to enhance treatment options, including novel therapies and gene therapies, create opportunities for companies to offer innovative solutions. Initiatives to raise awareness about classical homocystinuria on a global scale open the door for market players to expand their reach and contribute to early diagnosis and treatment. Strategic collaborations between pharmaceutical companies, research institutions, and healthcare organizations can facilitate the development and commercialization of new therapies fostering market growth of classical homocystinuria. Untapped markets in developing regions provide opportunities for market entry and expansion, particularly as healthcare infrastructure and awareness improve. The integration of advanced technologies, such as precision medicine and personalized therapies., can drive market growth by offering more targeted and effective treatment options.
Global Classical Homocystinuria Market – Geographical Insight
The market for classical homocystinuria is segmented into regions such as North America, Latin America, Europe, Asia-Pacific, the Middle East & Africa. North America is the largest market for global classical homocystinuria due to the forefront of classical homocystinuria research and development, with numerous clinical trials and innovative therapies in progress. European countries have a well-established regulatory framework supporting the development and approval of orphan drugs, providing a conducive environment for classical homocystinuria treatment advancement. Asia-Pacific’s increasing awareness, improving healthcare infrastructure, and growing patient population contribute to the emergence of Asia-Pacific as a potential market for classical homocystinuria treatments.
Global Classical Homocystinuria Market – Key Development
Research Methodology: Aspects
Market research is a crucial tool for organizations aiming to navigate the dynamic landscape of customer preferences, business trends, and competitive landscapes. At Cognizance Market Research, acknowledging the importance of robust research methodologies is vital to delivering actionable insights to our clientele. The significance of such methodologies lies in their capability to offer clarity in complexity, guiding strategic management with realistic evidence rather than speculation. Our clientele seek insights that excel superficial observations, reaching deep into the details of consumer behaviours, market dynamics, and evolving opportunities. These insights serve as the basis upon which businesses craft tailored approaches, optimize product offerings, and gain a competitive edge in an ever-growing marketplace.
The frequency of information updates is a cornerstone of our commitment to providing timely, relevant, and accurate insights. Cognizance Market Research adheres to a rigorous schedule of data collection, analysis, and distribution to ensure that our reports reflect the most current market realities. This proactive approach enables our clients to stay ahead of the curve, capitalize on emerging trends, and mitigate risks associated with outdated information.
Our research process is characterized by meticulous attention to detail and methodological rigor. It begins with a comprehensive understanding of client objectives, industry dynamics, and research scope. Leveraging a combination of primary and secondary research methodologies, we gather data from diverse sources including surveys, interviews, industry reports, and proprietary databases. Rigorous data analysis techniques are then employed to derive meaningful insights, identify patterns, and uncover actionable recommendations. Throughout the process, we remain vigilant in upholding the highest standards of data integrity, ensuring that our findings are robust, reliable, and actionable.
Key phases involved in in our research process are mentioned below:

Understanding Clients’ Objectives:
Extensive Discussions and Consultations:
Industry and Market Segment Analysis:
Target Audience Understanding:
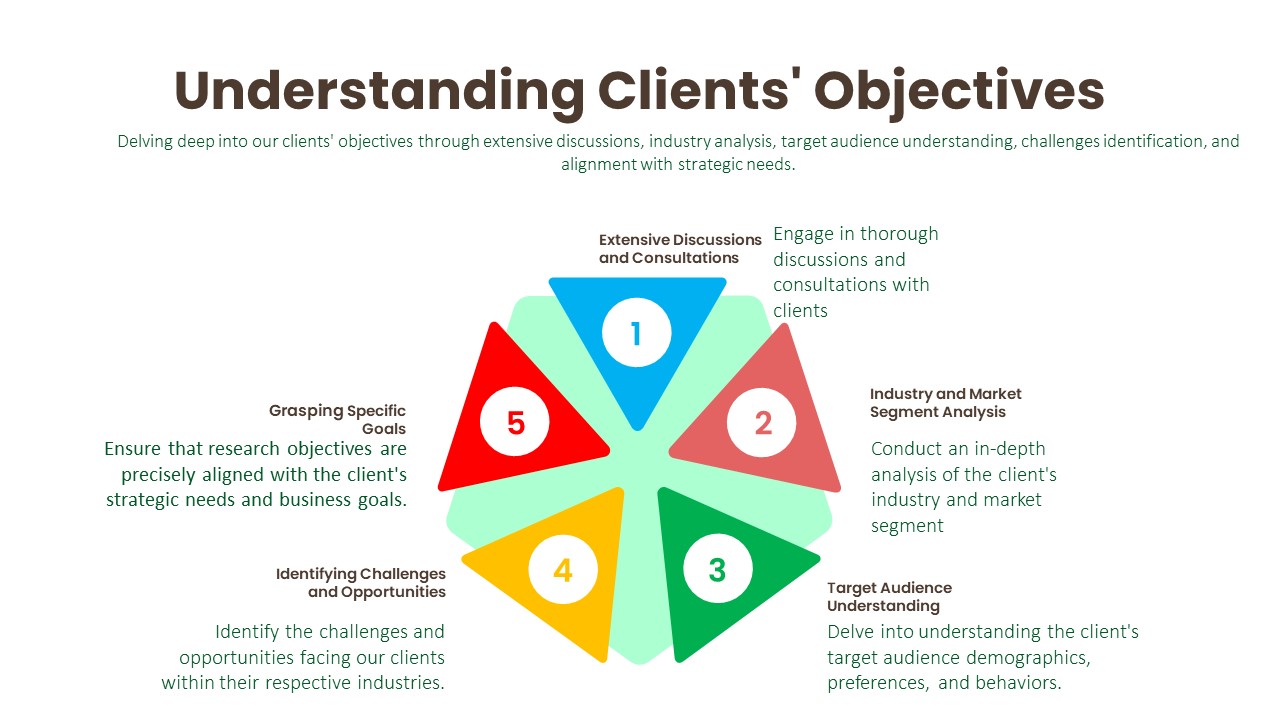
Identifying Challenges and Opportunities:
Grasping Specific Goals:
Data Collection:
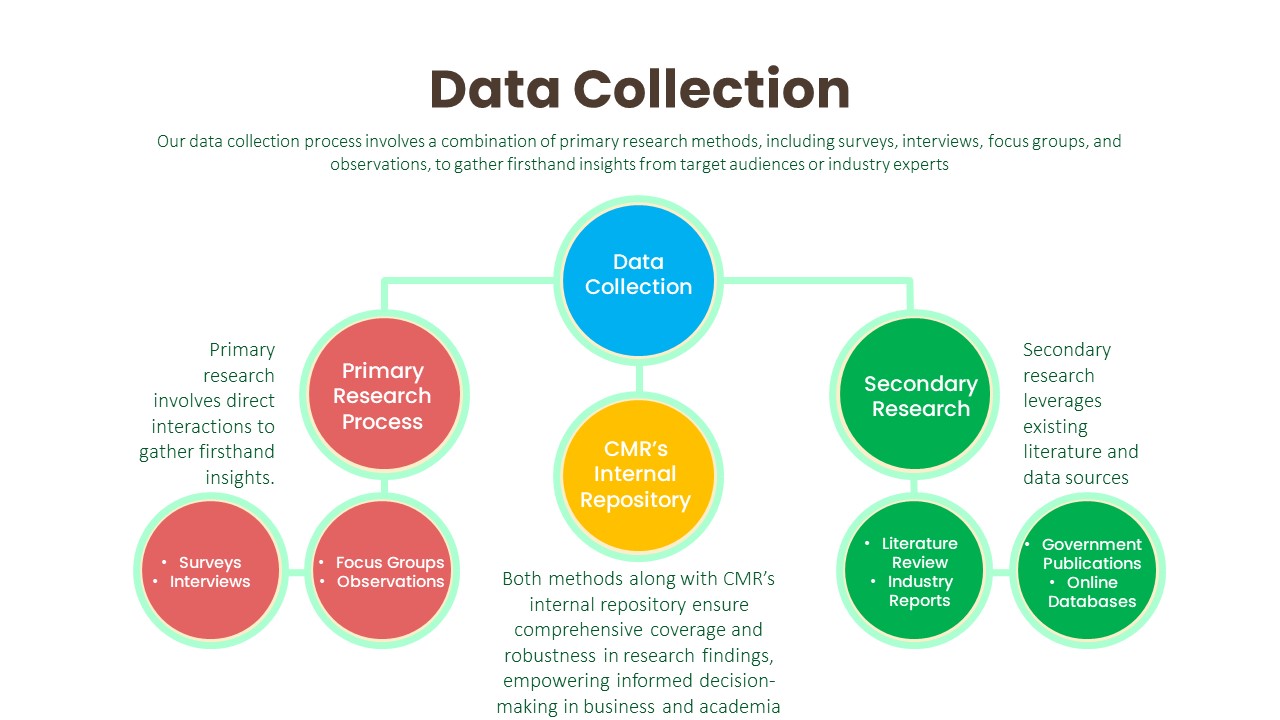
Primary Research Process:
Secondary Research Process:
Data Analysis:
The data analysis phase serves as a critical juncture where raw data is transformed into actionable insights that inform strategic decision-making. Through the utilization of analytical methods such as statistical analysis and qualitative techniques like thematic coding, we uncover patterns, correlations, and trends within the data. By ensuring the integrity and validity of our findings, we strive to provide clients with accurate and reliable insights that accurately reflect the realities of the market landscape.

Transformation of Raw Data:
Utilization of Analytical Methods:
Statistical Analysis:
Qualitative Analysis Techniques:
Integrity and Validity Maintenance:
Data Validation:
The final phase of our research methodology is data validation, which is essential for ensuring the reliability and credibility of our findings. Validation involves scrutinizing the collected data to identify any inconsistencies, errors, or biases that may have crept in during the research process. We employ various validation techniques, including cross-referencing data from multiple sources, conducting validity checks on survey instruments, and seeking feedback from independent experts or peer reviewers. Additionally, we leverage internal quality assurance protocols to verify the accuracy and integrity of our analysis. By subjecting our findings to rigorous validation procedures, we instill confidence in our clients that the insights they receive are robust, reliable, and trustworthy.
Importance of Data Validation:
Scrutiny of Collected Data:
Validation Techniques:
Internal Quality Assurance Protocols:
We can customize every report – free of charge – including purchasing stand-alone sections or country-level reports
We help clients to procure the report or sections of the report at their budgeted price. Kindly click on the below to avail

Cognizance market research is continuously guiding customers around the globe towards strategies for transformational growth. Today, businesses have to innovate more than ever before, not just to survive, but to succeed in the future

© 2023 All rights Reserved. Cognizance Market Research