
Biopharmaceutical logistics encompasses the intricate processes involved in the transportation and distribution of biopharmaceutical products from manufacturers to end-users. This specialized branch of logistics is characterized by the unique challenges posed by the nature of biopharmaceuticals, which often include sensitive and high-value medical goods like vaccines and biologics. One of the central concerns in biopharmaceutical logistics is maintaining the integrity and efficacy of these products, which are often temperature-sensitive. As a result, the logistics chain integrates advanced technologies, such as cold chain management systems, to ensure that products are stored and transported within specified temperature ranges throughout the supply chain. Furthermore, compliance with stringent regulatory standards is a fundamental aspect of biopharmaceutical logistics. Adherence to good distribution practices (GDP) and good manufacturing practices (GMP) is essential to uphold the quality and safety of the products. Robust tracking and traceability systems are implemented to monitor the movement of biopharmaceuticals at each stage, facilitating rapid identification and resolution of any deviations or issues that may arise.
Packing is another critical element in biopharmaceutical logistics, requiring specialized containers and materials to protect products from external factors like temperature fluctuations, shocks, and vibrations. This becomes particularly crucial when transporting biopharmaceuticals across borders, where diverse climates and handling procedures come into play. In essence, biopharmaceutical logistics is a highly specialized field that demands precision, compliance with regulatory standards, and the integration of cutting-edge technologies.
Global Biopharmaceutical Logistics Market – Competitive Landscape
On December 4, 2023, Sara Nayeem, M.D., an experienced biopharmaceutical venture investor, joined Enavate Sciences as executive vice president of investment. On August 21, 2023, Andreessen Co-leads USD 200 million investment in Biotech startup Genesis Therapeutics. On June 1, 2023, Invox Pharma increased investment in pHion Therapeutics to develop an mRNA vaccine. On November 24, 2022, Fujifilm invested USD 188 million in a new North Carolina cell culture media facility.
Some of the Key Players in the Global Biopharmaceutical Logistics Market Include –
Global Biopharmaceutical Logistics Market – Growth Drivers
The continuous growth of the biopharmaceutical industry driven by advancements in biotechnology and the increasing prevalence of complex diseases, serves as a fundamental driver for the expansion of biopharmaceutical logistics. According to the National Center for Biotechnology Information (NCBI), the prevalence rates of complex diseases are between 54 and 214 per 100,000 population. The growing demand for specialty pharmaceuticals, including biologics and personalized medicines, fuels the need for specialized logistics services capable of handling the unique storage and transportation requirements of these products. The global expansion of clinic trials, particularly in diverse geographic regions, creates opportunities for biopharmaceutical logistics to support the efficient and secure movement of investigational drugs and trial-related materials. Continuous advancements in logistic technologies, such as IoT-enabled real-time monitoring, blockchain for supply chain transparency, and data analytics, drive efficiency improvement and enhance the reliability of biopharmaceutical logistics. Collaborative efforts and strategic alliances within the biopharmaceutical supply chain, including partnerships between logistics providers manufacturers, and distributors, contribute to streamlined operation and improved logistics capabilities. Increased healthcare expenditure globally, coupled with expanding access to advanced therapies, supports the growth of biopharmaceutical logistics by creating a larger market for the distribution of pharmaceutical products.
Global Biopharmaceutical Logistics Market – Restraint
The highly regulated nature of the biopharmaceutical industry poses a restraint on logistics, poses a restraint on logistics, requiring compliance with complex and evolving global regulations, which can lead to increased operational challenges. The need for precise temperature control during transportation and storage imposes constraints on logistics, as maintaining the required conditions, especially for biologics, often requires sophisticated and costly cold chain infrastructure. According to Statista biopharmaceutical industry’s spending on cold chain logistics is projected to reach nearly USD 19 billion. Due to the high value and sensitivity of biopharmaceuticals, logistics face challenges related to security, including the risk of theft, counterfeiting, and tampering, necessitating robust security measures. Cross-border logistics encounter challenges related to customs procedures and import restrictions, which vary between countries, leading to delays and increased complexity in the movement of biopharmaceuticals. The specialized handling and transportation requirements of biopharmaceuticals contribute to elevated operational costs for logistics providers, impacting profit margins and requiring continuous investment in technology and infrastructure. Expanding into emerging markets can be challenging due to logistical complexities, diverse regulatory landscapes, and infrastructure limitations, hindering the seamless distribution of biopharmaceuticals. The limited shelf life of some biopharmaceutical products adds pressure on logistics to ensure timely delivery, minimizing the risk of product expiration and wastage. Certain biopharmaceuticals may have specific transportation mode requirements, limiting the flexibility of logistic providers and potentially causing delays or increased costs when specialized transport is needed.
Global Biopharmaceutical Logistics Market -Opportunities
The globalization of clinical trials creates opportunities for biopharmaceutical logistics to support the transportation of investigational borders, requiring expertise in compliance and secure handling. Developing efficient last-mile delivery solutions, especially in urban areas, presents opportunities for logistic providers to address the unique challenges of delivering biopharmaceuticals to healthcare facilities and end-users. The increasing focus on sustainability offers opportunities for logistics providers to implement and market environmentally friendly practices, aligning with the industry’s growing emphasis on corporate responsibility. Leveraging data analytics for route
Global Biopharmaceutical Logistics Market – Geographical Insight
The global biopharmaceutical logistic market is segmented into regions such as North America, Latin America, Europe, Asia-Pacific, the Middle East & Africa. North America is the largest market for biopharmaceutical logistics due to extensive logistic infrastructure facilitating rapid distribution, adherence to quality standards, and documentation throughout the supply chain. Asia-Pacific is the growing market for biopharmaceutical logistics due to distinct requirements for the transportation and storage of biopharmaceuticals. European regulations set comprehensive standards for biopharmaceutical logistics emphasizing traceability and compliance with good distribution practices.
Global Biopharmaceutical Logistics Market – Key Development
Research Methodology: Aspects
Market research is a crucial tool for organizations aiming to navigate the dynamic landscape of customer preferences, business trends, and competitive landscapes. At Cognizance Market Research, acknowledging the importance of robust research methodologies is vital to delivering actionable insights to our clientele. The significance of such methodologies lies in their capability to offer clarity in complexity, guiding strategic management with realistic evidence rather than speculation. Our clientele seek insights that excel superficial observations, reaching deep into the details of consumer behaviours, market dynamics, and evolving opportunities. These insights serve as the basis upon which businesses craft tailored approaches, optimize product offerings, and gain a competitive edge in an ever-growing marketplace.
The frequency of information updates is a cornerstone of our commitment to providing timely, relevant, and accurate insights. Cognizance Market Research adheres to a rigorous schedule of data collection, analysis, and distribution to ensure that our reports reflect the most current market realities. This proactive approach enables our clients to stay ahead of the curve, capitalize on emerging trends, and mitigate risks associated with outdated information.
Our research process is characterized by meticulous attention to detail and methodological rigor. It begins with a comprehensive understanding of client objectives, industry dynamics, and research scope. Leveraging a combination of primary and secondary research methodologies, we gather data from diverse sources including surveys, interviews, industry reports, and proprietary databases. Rigorous data analysis techniques are then employed to derive meaningful insights, identify patterns, and uncover actionable recommendations. Throughout the process, we remain vigilant in upholding the highest standards of data integrity, ensuring that our findings are robust, reliable, and actionable.
Key phases involved in in our research process are mentioned below:

Understanding Clients’ Objectives:
Extensive Discussions and Consultations:
Industry and Market Segment Analysis:
Target Audience Understanding:
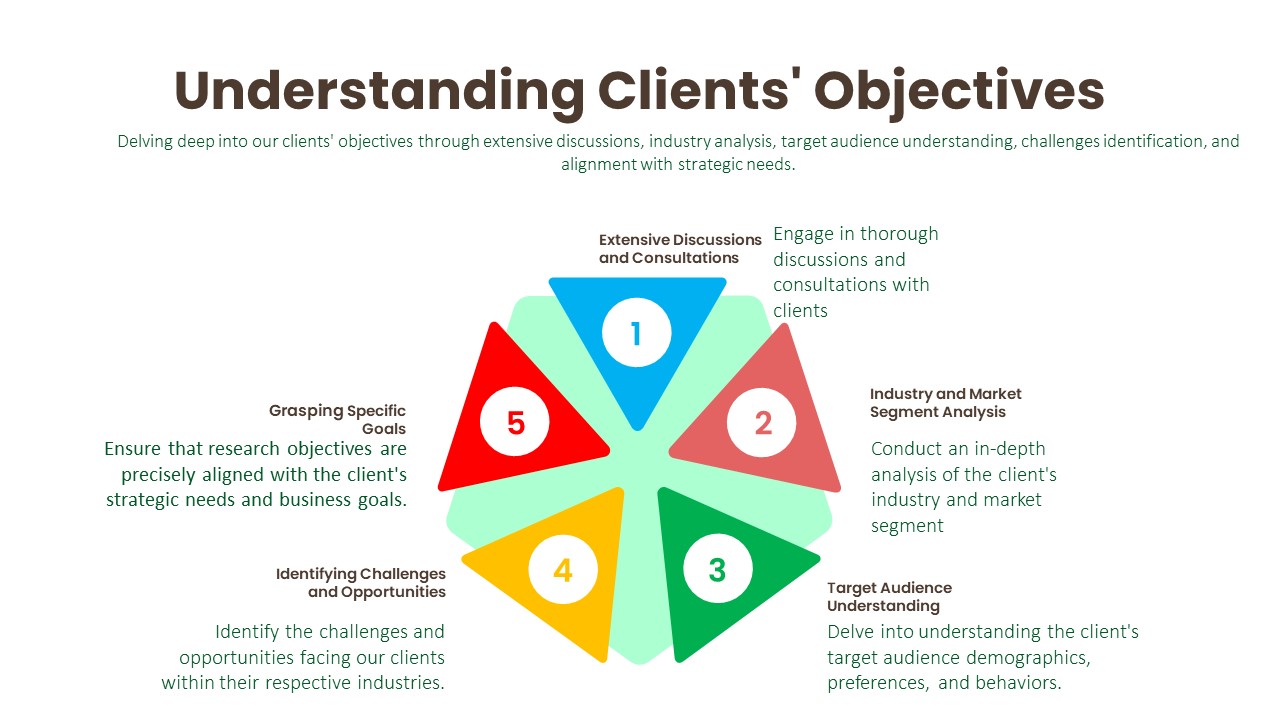
Identifying Challenges and Opportunities:
Grasping Specific Goals:
Data Collection:
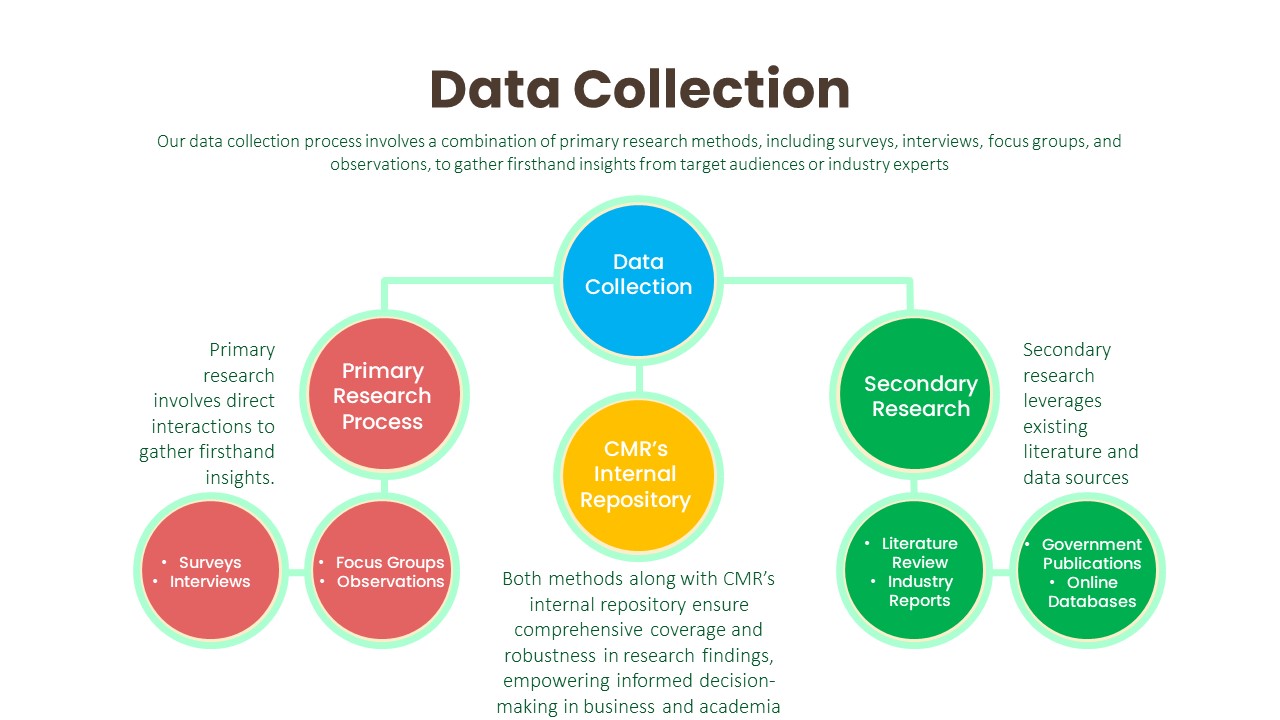
Primary Research Process:
Secondary Research Process:
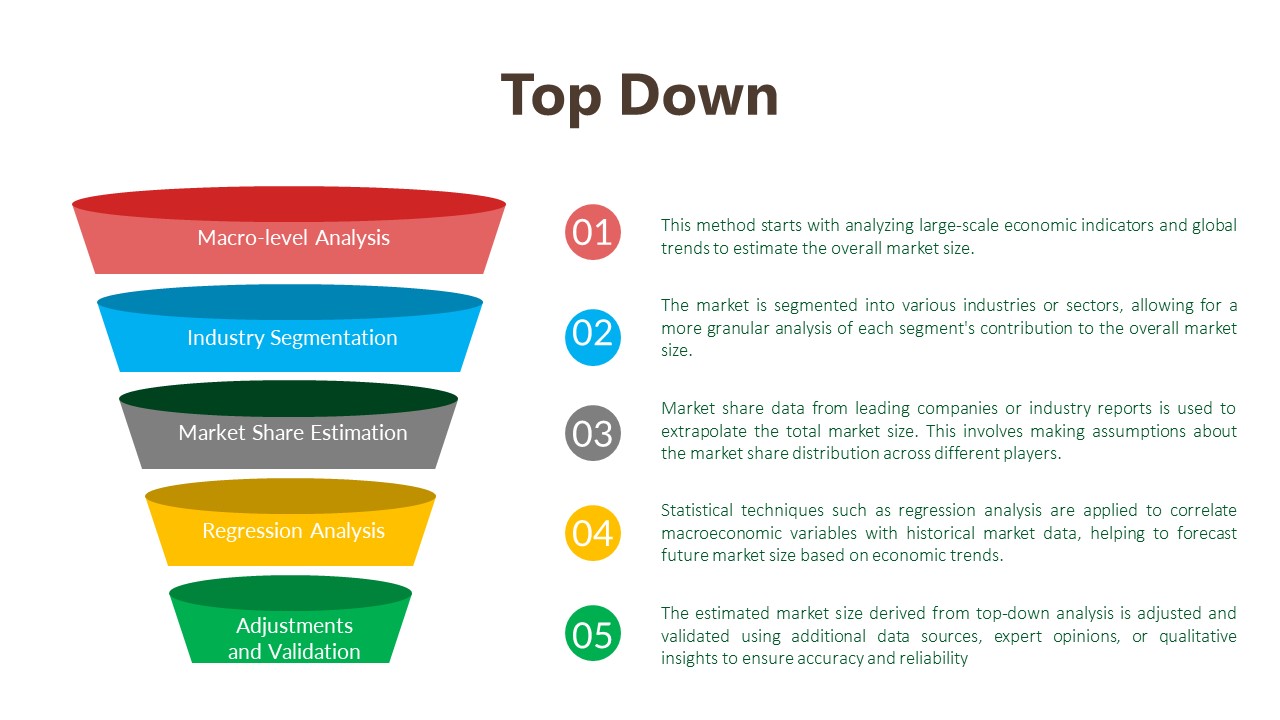
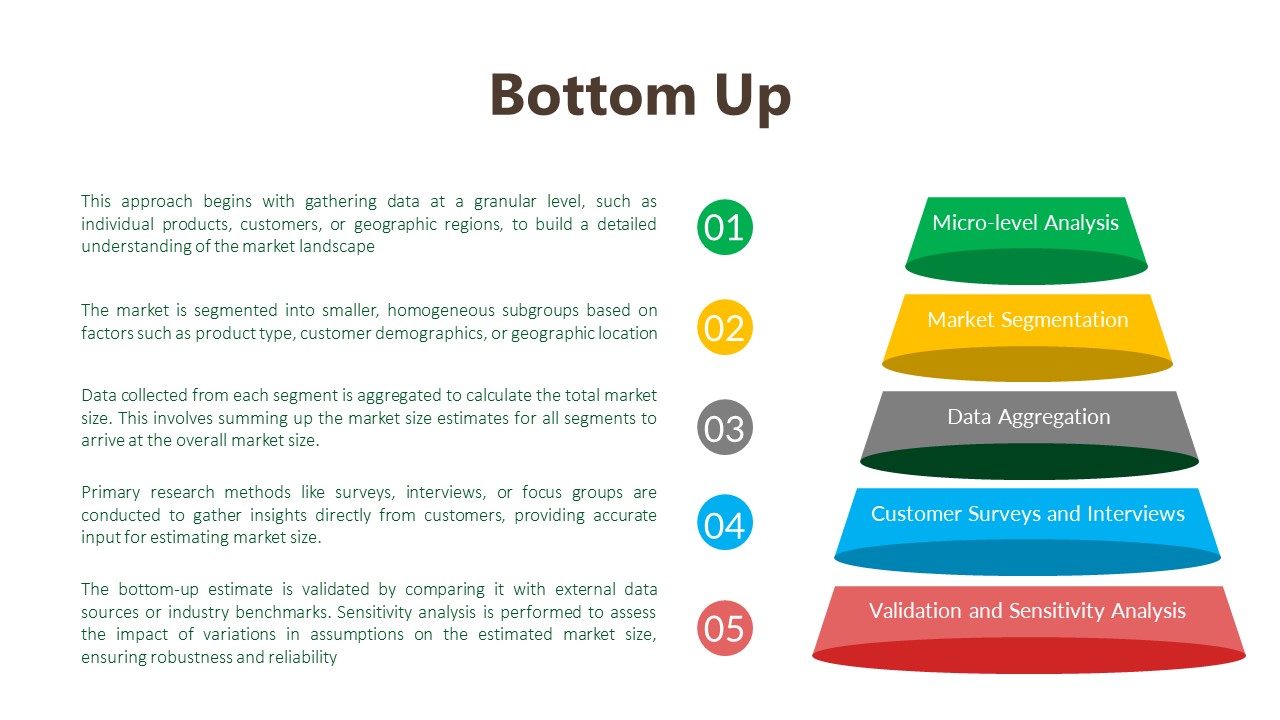
Data Analysis:
The data analysis phase serves as a critical juncture where raw data is transformed into actionable insights that inform strategic decision-making. Through the utilization of analytical methods such as statistical analysis and qualitative techniques like thematic coding, we uncover patterns, correlations, and trends within the data. By ensuring the integrity and validity of our findings, we strive to provide clients with accurate and reliable insights that accurately reflect the realities of the market landscape.

Transformation of Raw Data:
Utilization of Analytical Methods:
Statistical Analysis:
Qualitative Analysis Techniques:
Integrity and Validity Maintenance:
Data Validation:
The final phase of our research methodology is data validation, which is essential for ensuring the reliability and credibility of our findings. Validation involves scrutinizing the collected data to identify any inconsistencies, errors, or biases that may have crept in during the research process. We employ various validation techniques, including cross-referencing data from multiple sources, conducting validity checks on survey instruments, and seeking feedback from independent experts or peer reviewers. Additionally, we leverage internal quality assurance protocols to verify the accuracy and integrity of our analysis. By subjecting our findings to rigorous validation procedures, we instill confidence in our clients that the insights they receive are robust, reliable, and trustworthy.
Importance of Data Validation:
Scrutiny of Collected Data:
Validation Techniques:
Internal Quality Assurance Protocols:
We can customize every report – free of charge – including purchasing stand-alone sections or country-level reports
We help clients to procure the report or sections of the report at their budgeted price. Kindly click on the below to avail

Cognizance market research is continuously guiding customers around the globe towards strategies for transformational growth. Today, businesses have to innovate more than ever before, not just to survive, but to succeed in the future

© 2023 All rights Reserved. Cognizance Market Research