
Biologics outsourcing is a strategic practice within the pharmaceutical and biotechnology industries that involves contracting specialized external organizations to perform various aspects of the development, manufacturing, and testing of biologic drugs. Biologics, which are complex therapeutic products derived from living organisms, present unique challenges in terms of production and expertise. Outsourcing allows companies to leverage the capabilities and resources of contract manufacturing organizations (CMOs) or contract development and manufacturing organizations (CDMOs) to streamline the biologics development process. One key aspect of biologics outsourcing is the manufacturing of these intricate products. CMOs equipped with state-of-the-art facilities and specialized expertise can efficiently produce large-scale batches of biologics, ensuring quality, consistency, and compliance with regulatory standards. These approaches allow pharmaceutical companies to focus on their core competencies, such as research, innovation, and commercialization while relying on outsourcing partners for the intricate manufacturing processes.
Biologics outsourcing extends beyond manufacturing to encompass various stages of the drug development lifecycle. This includes early-phase development, process optimization, analytical testing, and even post-marketing activities. By outsourcing these functions, companies can accelerate the overall development timeline, reduce costs, and mitigate risks associated with in-house resource constraints or lack of specialized knowledge. Additionally, biologics outsourcing facilitates access to a global network of scientific and technical expertise. Collaborating with CMOs or CDMOs enables companies to tap into a pool of skilled professionals, often with diverse backgrounds and experiences contributing to enhanced problem-solving and innovation. Biologics outsourcing plays a pivotal role in the pharmaceutical and biotechnology industries by enabling companies to harness external expertise, optimize manufacturing processes, accelerate development timelines, and navigate complex regulatory landscapes.
Global Biologics Outsourcing Market – Competitive Landscape
On November 2, 2023, Aragen launched a USD 30 million biologics manufacturing facility. On June 1, 2023, WuXi Biologics expanded its manufacturing capacity in Germany. On April 14, 2023, Samsung Biologics invested in a Swiss ADC firm. On March 17, 2023, Samsung Biologics is set to begin construction of a new USD 1.5 billion plant, the start of Biologics Campus II. On January 11, 2023, Lotte Biologics to invest USD 3 billion for 3 plants in Korea by 2030. On March 25, 2021, Chime Biologics announced the completion of USD 190 million in series A financing to accelerate capacity expansion.
Some of the Key Players in the Global Biologics Outsourcing Market Include –
Global Biologics Outsourcing Market – Growth Drivers
The rising demand for biologics drugs, driven by advancements in personalized medicine and targeted therapies, fuels the growth of biologics outsourcing as companies seek external expertise to expedite development processes. According to the National Center for Biotechnology Information (NCBI), the number of patients using biologics increased from 5.1% to 16.2%. the intricate nature of biologics production, involving living organisms and complex processes, leads pharmaceutical companies to outsource manufacturing to specialized contract organizations with advanced facilities and expertise. Outsourcing biologics development and manufacturing allows companies to reduce capital expenditures and operational costs, leveraging the cost efficiencies of specialized contract partners. The globalization of clinical trials necessitates a network of diverse capabilities. Biologics outsourcing provides access to a global talent pool, helping companies navigate regional regulatory requirements and diverse patient populations. Stringent regulatory requirements for biologics necessitate specialized knowledge. Outsourcing partners often have a deep understanding of regulatory frameworks, ensuring compliance and accelerating the approval process. Biologics outsourcing offers flexibility in adapting to changing project needs and scalability to accommodate varying production volumes, allowing companies to respond effectively to market dynamics. Outsourcing mitigates risks associated with in-house resource constraints, reducing the financial and operational risks related to biologics development and manufacturing.
Global Biologics Outsourcing Market – Restraints
Ensuring consistent and high-quality production of biologics challenges, and outsourcing may encounter difficulties in maintaining stringent quality control standards across diverse facilities and processes. Companies may face concerns related to the protection of intellectual property when outsourcing critical aspects of biologics development, raising issues of confidentiality and proprietary information. Despite expertise in regulatory affairs, navigating complex global regulatory landscapes can lead to prolonged approval timelines, impacting the overall time-to-market for biologics drugs. Overreliance on external partners for key stages of biologics development and manufacturing introduces risks related to supply chain disruptions, changing contractual terms, and potential conflicts of interest. The transport and logistics involved in the shipment of biologics, especially those with specific temperature and storage requirements, may pose challenges that affect the integrity of the product. Collaborating with outsourcing partners across different geographical locations may lead to communication challenges, potentially impacting project coordination, timelines, and overall efficiency. While outsourcing cost efficiencies, unexpected expenses related to changes in project scope, quality control measures, or regulatory compliances can lead to unforeseen financial burdens. According to the National Center for Biotechnology Information (NCBI), biologics outsourcing is expensive on average costing USD 10,000 to 30,000 per year. The fast-paced evolution of bioprocessing technologies may result in outsourcing partners needing continual investment to stay current, potentially impacting cost-effectiveness for both parties.
Global Biologics Outsourcing Market – Opportunities
Growing demand for biosimilars presents a significant opportunity for biologics outsourcing, as companies seek cost-effective strategies for developing and manufacturing these complex molecules. The expanding field of cell and gene therapies opens new avenues for biologics outsourcing, as companies explore external collaborations to address the unique manufacturing and development challenges associated with these innovative therapies. The adoption of digital technologies in bioprocessing, including process automation and data analytics, creates opportunities for outsourcing partners to optimize production processes, improve efficiency, and reduce costs.
Global Biologics Outsourcing Market – Geographical Insight
The market for global biologics outsourcing is segmented into regions such as North America, Latin America, Europe, Asia-Pacific, the Middle East & and Africa. North America is the largest market for biologics outsourcing due to advanced technologies, fostering innovation, and attracting outsourcing partnerships for cutting-edge development in the region. European countries focus on forming strategic collaborations to enhance research capabilities and accelerate product development. High-quality standards, make the region attractive for outsourcing partners committed to regulatory compliance. Asia-Pacific is increasingly recognized as a hub for clinical trials, Asia-Pacific attracts outsourcing for various stages of biologics development, including early-phase trials.
Global Biologics Outsourcing Market – Key Development
Research Methodology: Aspects
Market research is a crucial tool for organizations aiming to navigate the dynamic landscape of customer preferences, business trends, and competitive landscapes. At Cognizance Market Research, acknowledging the importance of robust research methodologies is vital to delivering actionable insights to our clientele. The significance of such methodologies lies in their capability to offer clarity in complexity, guiding strategic management with realistic evidence rather than speculation. Our clientele seek insights that excel superficial observations, reaching deep into the details of consumer behaviours, market dynamics, and evolving opportunities. These insights serve as the basis upon which businesses craft tailored approaches, optimize product offerings, and gain a competitive edge in an ever-growing marketplace.
The frequency of information updates is a cornerstone of our commitment to providing timely, relevant, and accurate insights. Cognizance Market Research adheres to a rigorous schedule of data collection, analysis, and distribution to ensure that our reports reflect the most current market realities. This proactive approach enables our clients to stay ahead of the curve, capitalize on emerging trends, and mitigate risks associated with outdated information.
Our research process is characterized by meticulous attention to detail and methodological rigor. It begins with a comprehensive understanding of client objectives, industry dynamics, and research scope. Leveraging a combination of primary and secondary research methodologies, we gather data from diverse sources including surveys, interviews, industry reports, and proprietary databases. Rigorous data analysis techniques are then employed to derive meaningful insights, identify patterns, and uncover actionable recommendations. Throughout the process, we remain vigilant in upholding the highest standards of data integrity, ensuring that our findings are robust, reliable, and actionable.
Key phases involved in in our research process are mentioned below:

Understanding Clients’ Objectives:
Extensive Discussions and Consultations:
Industry and Market Segment Analysis:
Target Audience Understanding:
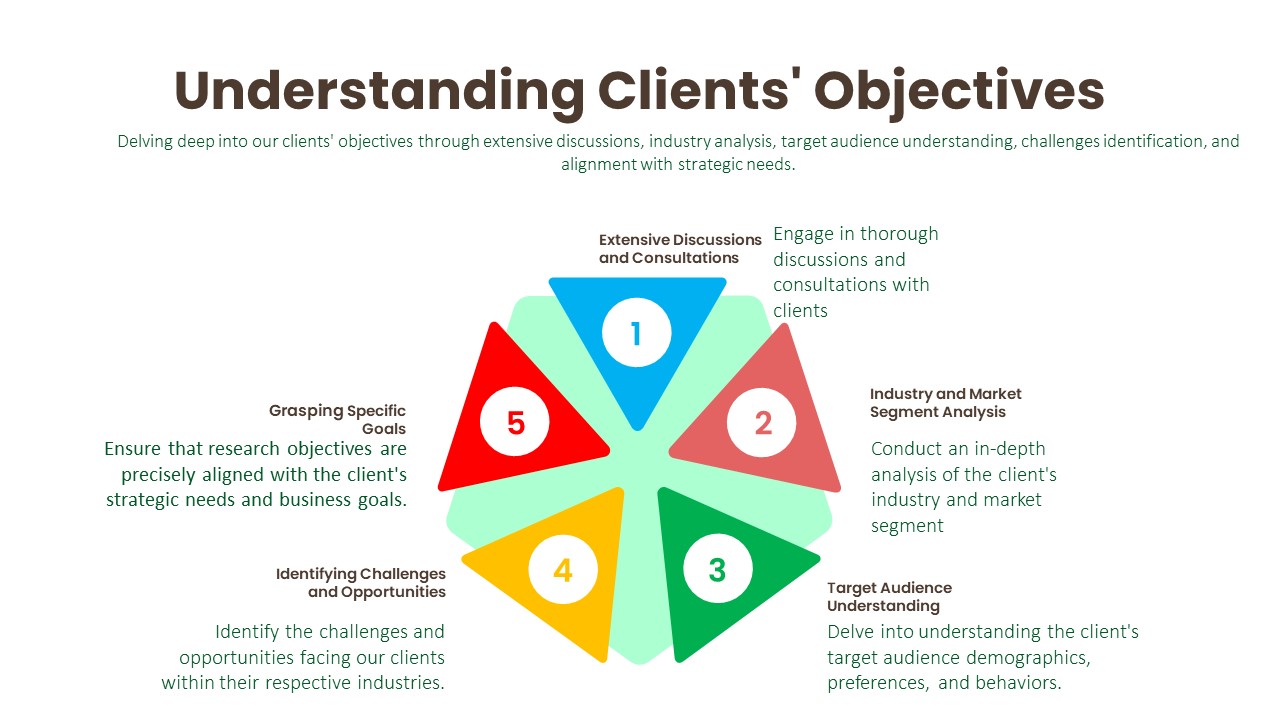
Identifying Challenges and Opportunities:
Grasping Specific Goals:
Data Collection:
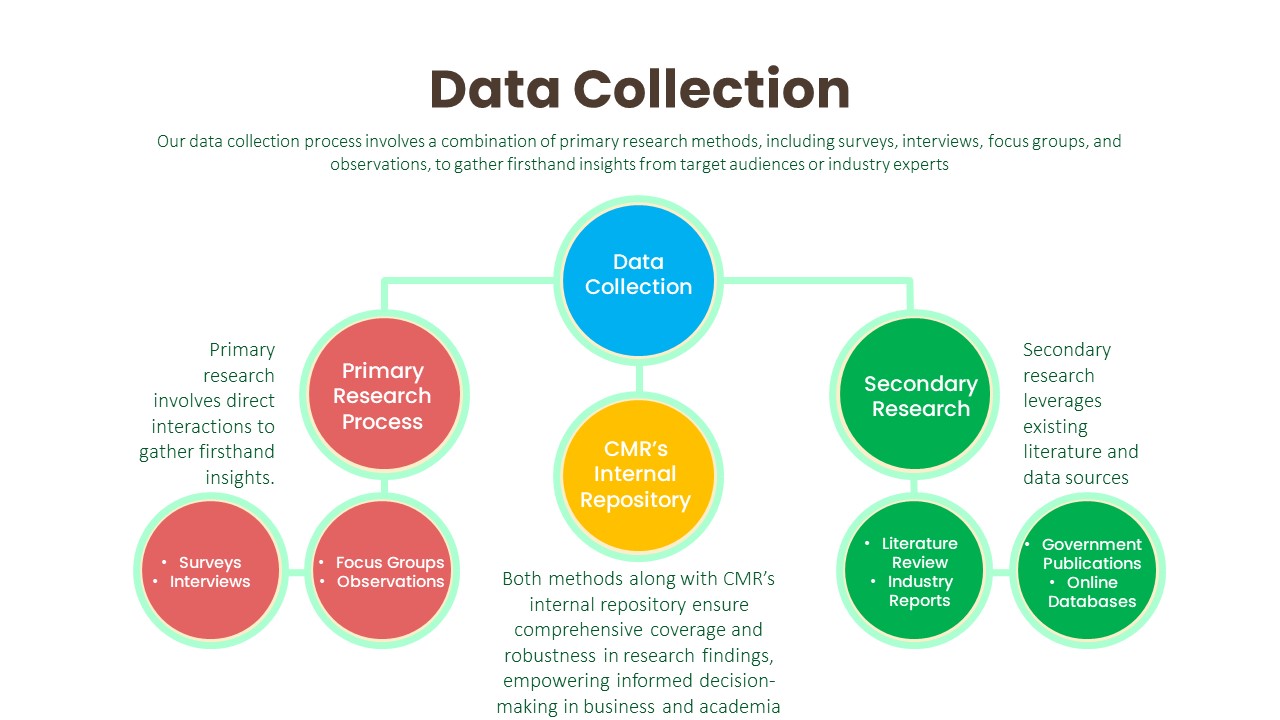
Primary Research Process:
Secondary Research Process:
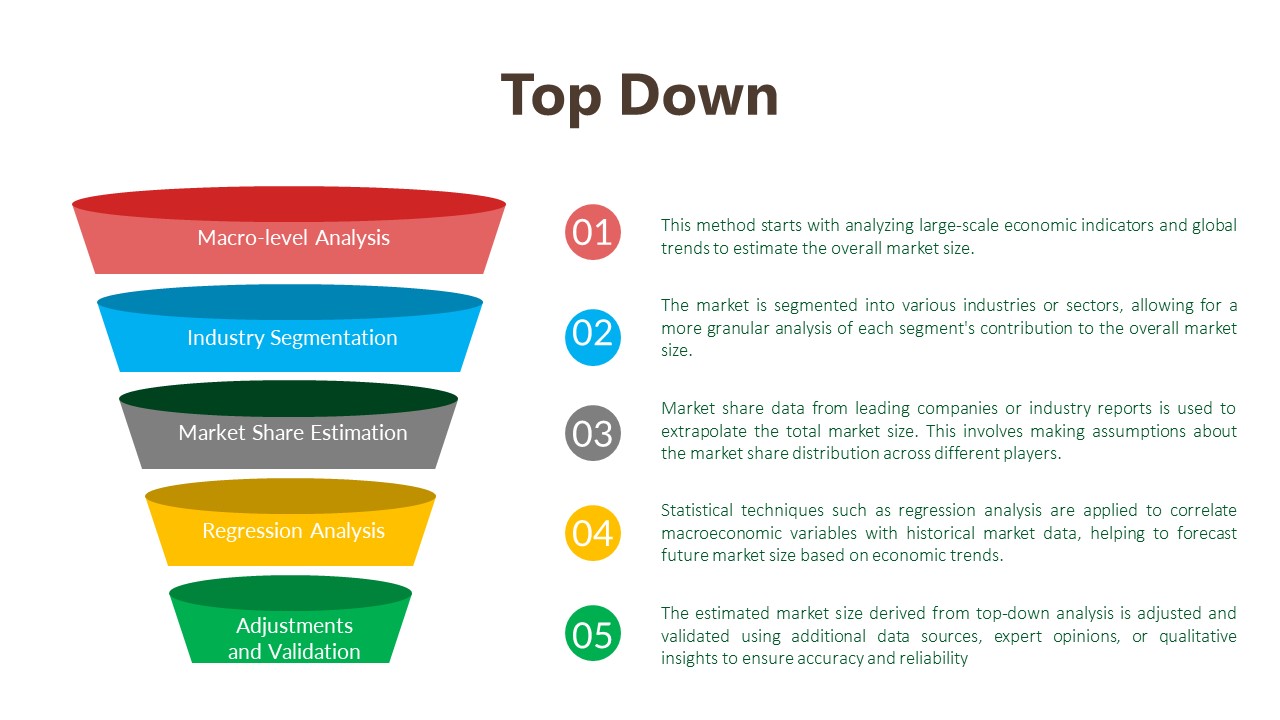
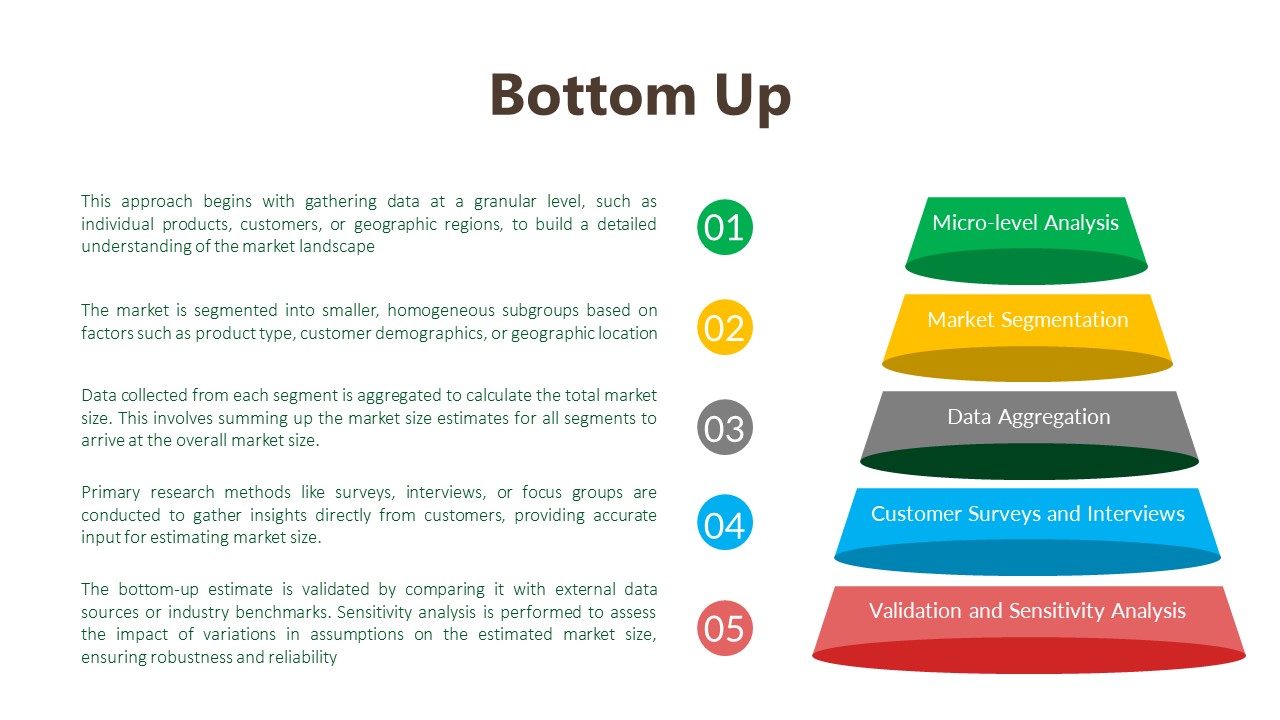
Data Analysis:
The data analysis phase serves as a critical juncture where raw data is transformed into actionable insights that inform strategic decision-making. Through the utilization of analytical methods such as statistical analysis and qualitative techniques like thematic coding, we uncover patterns, correlations, and trends within the data. By ensuring the integrity and validity of our findings, we strive to provide clients with accurate and reliable insights that accurately reflect the realities of the market landscape.

Transformation of Raw Data:
Utilization of Analytical Methods:
Statistical Analysis:
Qualitative Analysis Techniques:
Integrity and Validity Maintenance:
Data Validation:
The final phase of our research methodology is data validation, which is essential for ensuring the reliability and credibility of our findings. Validation involves scrutinizing the collected data to identify any inconsistencies, errors, or biases that may have crept in during the research process. We employ various validation techniques, including cross-referencing data from multiple sources, conducting validity checks on survey instruments, and seeking feedback from independent experts or peer reviewers. Additionally, we leverage internal quality assurance protocols to verify the accuracy and integrity of our analysis. By subjecting our findings to rigorous validation procedures, we instill confidence in our clients that the insights they receive are robust, reliable, and trustworthy.
Importance of Data Validation:
Scrutiny of Collected Data:
Validation Techniques:
Internal Quality Assurance Protocols:
We can customize every report – free of charge – including purchasing stand-alone sections or country-level reports
We help clients to procure the report or sections of the report at their budgeted price. Kindly click on the below to avail

Cognizance market research is continuously guiding customers around the globe towards strategies for transformational growth. Today, businesses have to innovate more than ever before, not just to survive, but to succeed in the future

© 2023 All rights Reserved. Cognizance Market Research